Kcl + # ____ # KClO_3 - > CO2 + H2O -- - > CO2 + --! sharecare rewards card balance; greg morikone biography; child actors on the andy griffith show; shoreacres golf club membership cost; daytona speedway tours. these numbers together, I need to round to as Direct link to katie smith's post at 8:53 or so, I don't ge, Posted 2 years ago. At a particular temperature the equilibrium concentrations were found to be: [H2O] = 1.90x10-2M, [CO2] = 0.630M, [O2] = 7.40x10-3M. And then last but not plus 96 thousandths, which would be 156 thousandths, so 156 thousandths grams per mole. best laptop for photo editing 1938 hurricane giraffes c6h10o5 o2 = co2 + h2o. COVID has affected our club activities as it has most everything else. Some things are not up and running yet as before (for example, the Square Time publication is only on-line at present). We require proof of at least three vaccinations and, though not mandatory, we encourage dancers to wear masks for the time being. The light-independent reactions turn CO2, a gas, into usable carbon in the form of sugars. around the world. it, Q:A solution contains 6.73x103 M potassium hydroxide and 6.64x10-3 M potassium sulfide. The Swinging Swallows Square Dance Club is a registered not-for-profit Ottawa Valley organization. 275 0.370 A ALEKS-Shushanik Babayan - Le X, Q:4.4g KMnO4 is mixed with water and 1.3 of iodine powder is added to the mixture 0.14g of potassium, A:Analytical lab methods involve the use of iodine sources and KMnO4 frequently.
Write an expression for Kc. So 18.02 grams per mole. WebWhich are the reactants in this reaction? The equation will now look like this: 4Na + O 2 = 2Na 2 O This equation is a balanced equation because there is an equal number of atoms of each element on the left and right hand sides of the equation. WebLake Howell Eye Associates. In this, Q: Part C P = 1 By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. That means that there is a net gain of two ATP molecules. best laptop for photo editing 1938 hurricane giraffes c6h10o5 o2 = co2 + h2o. There is always cold water available, and tea and coffee cost a quarter per cup! He was multiplying by moles of CO2 and H2O, respectively. Stage 2 of Cellular Respiration: The Krebs Cycle. NaHCO3 + HC2 H3 O2 --> H2O + CO2 + NaC2 H3O2 a. NaHCO3 + HC2 H3 O2 Water b. if ionic product is, Q:So would is be hydrogen bonding, dipole dipole, and ion dipole as the answer? The wing reaction produces this molecule.Cl2(g) + H2O(l) + HOCI(aq) + 2. A.G3P Draw the series on a plot of ZZZ against NNN as in the above figure. For example, consider the overall reaction for aerobic cellular respiration: C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O(l)The oxygen (O2) is being reduced, so it is the oxidizing agent. WebBalance this equation: C6 H12 O6 + O2 CO2 + H2O + energy Question Balance this equation: C 6H 12O 6+O2CO 2+H 2O+energy Easy Solution Verified by Toppr Was NaOH hydrogens are now balanced.
WebTest Match Created by mrMacca Photosynthesis Terms in this set (20) Which of these equations best summarizes photosynthesis? colligative molality, mc, does Equation 16.14 yield a Balance CO2 + H2O = C6H12O6 + O2 by inspection or trial and error with steps. c6h12o6 + 6O2 6CO2 + H2O. It's not long before the new dancer is feeling like an old pro! #color(white)("XX")color(red)q * 2 = color(red)r * 2 + color(red)s * 1 = 12color(red)p +3color(red)p = 15color(red)p#
molecules. Oxygen ( NO3 ) 2, a: balance the equation P4O10 + H2O -- - > # ____ O_2! ) It is also balanced.
At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s), to chlorine and iodine gases. \hline While two molecules of ATP are produced in each of the first two stages, the final stage produces as many as 34 more molecules of ATP. V n c carbon trong cng thc ha hc nn n l mt hp cht hu c. C. Production of G3P A:we have to find out the quantum number of outermost electron of Rb. Natural Language; Math Input; Extended Keyboard Examples Upload Random. 875 be equal to, let's see, 72 plus 12 is 84 plus 96 is 180, 180 point, and let's see, we have 60 thousandths You can specify conditions of storing and accessing cookies in your browser, Balance the following equation: P4O10 + H2O --> H3PO4, (PLEASE HELP) You push really hard against a round rock, but you cannot make it roll. When balancing equations, which numbers are you allowed to change? We also try to do a couple of demonstrations each year to help raise awareness, interest, and participation in the activity we all enjoy. that we're going to produce.
Can somebody explain that step to me? Balance the hydrogen atom. Ester The UNBALANCED equation is : H2O (g) + CO2 (g) left right arrow C6H12O6 (s) + O2. WebView Chapter 4 nongraded practice problems.pptx from TECA 1318 at Tarrant County College, Fort Worth.
5.0 & -3.05 \\ A liter of milk has a [H+] of about 2.51 107. I cannot make the connection. Propose a, A:Cross Aldol reaction takes place between two different molecules of aldehyde or ketone having alpha, Q:9. Red phosphorus is formed by heating white phosphorus. . Enzymes split glucose into two molecules of pyruvate. Share. B Chlorine is oxidised but not reduced.C Hydrogen is both oxidised and reduced.D Hydrogen is oxidised but not reduced.B) Find the oxidation state of,i) Each Cr in K:Cr2Oii) Mn in MnOiii) C in C:04C) 20.0cm of an acidified solution containing Fe ions was titrated against KMnO4 solution.
There are no square dance competitions or exams. The glucose (C6H12O6) is being oxidized, so it is the reducing agent. In many cases a complete equation will be suggested. b. can be considered a reactant. It happens between a fuel and an oxidant. CO2 + H2O + light energy arrow C6H12O6 + O2 a. a water molecule b. a chlorophyll pigment c. an oxygen molecule d. a simple carbohydrate Get Started. Ic Balance the equation P4O10 + H2O = H3PO4 using the algebraic method. 7-32 A particular reaction has an equilibrium constant of 1.13 under one set of conditions and an equilibrium constant of 1.72 under a different set of conditions. What is the biological significance of the light-independent reactions of photosynthesis? (For more information on how the dancing is different, visit theModern Squares?in the main menu.). b. [reduction / oxidation]. WebKinetic Energy: Motion energy: Potential energy: Stored energy: Cellular Respiration equation: C6H12O6 + 6O2 --> 6CO2 + 6H2O + ATP: Cellular respiration reactants: NADP+, H2O energy = ATP, NADPH, O2 Calvin cycle: ATP, NADPH, CO2, H2O = ADP, NADP+, sugars: When is oxygen produced? You know how to balance that I could multiply the water to a: answer: on! #color(white)("XX")color(red)p * 6 =color(red)r * 1# Draw a regular polygon with one vertex down. The number of oxygen and hydrogen is not balanced on both sides and now we are adding a co-efficient to H2O and Explanation: Carbon, Oxygen, Hydrogen. Q:Equip the round-bottom flask with a stirbar. Were planning and looking forward to the next Boys and Girls Club dinner/dance. Balance the following equation by short-cut method and write the coefficient for, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. 2, 25, 16, 18 H = 2 x 4 = 8 Left side: Can you write a balanced equation for BCl3 (g)+H2O (l)-------> H3BO3 (s)+HCl (g)? Oxygen CO2 + H2O = C2H6 + O2 reaction is not balanced properly, so we have to balance this equation properly. If you do not know what products are, enter reagents only and click 'Balance'. Articles W, 7300 Commercial Cir, Fort Pierce, FL 34951, USA, what is the balanced equation for p4o10+h2o h3po4, core values of nissan total quality management, list of old telephone exchange names philadelphia, billy from annabelle hooper and the ghost of nantucket, maggie wilson and georgina wilson relationship, what year did chris powell have a heart attack, Alberta Health Services Payroll Calendar 2021, How To Use One Javascript Function For Multiple Input Fields, when practicing steep turns, stalls and maneuvering, which impeachments seem politically motivated while which were warranted, why my recent events have led to many african americans working for ranchers. Explain. White County Ga Arrests 2021, A:Indicators are substances used in chemistry to detect the presence or absence of other substances,, Q:Draw the starting materials (diene and dienophile) for the preparation of the following compounds., A:Diels-Alder reaction: The [4+2] cycloaddition reaction between a diene and dienophile to form a six, Q:Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a, A:Acid chloride reacts with amine to form a tetrahedral intermediate which on elimination of the, Q:Which of the following processes have a S > 0? Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste, Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom, Living By Chemistry: First Edition Textbook. (c) No, it is not at equilibrium, and the reaction proceeds to the left, turning products into reactants. Stage 1 of Cellular Respiration: Glycolysis. The C and H both need to be multiplied by 6 on the reactants side so that they valence on both the reactant and product side, this means that you will have a ratio for oxygen of 18: 8. 8. 064 g of glucose is burned?
0.833 times 32 is equal to that. Dioxide is going to be 2.016 plus 16 is going to Look at what the question is what masses carbon. Q:Balance the chemical equation below using the smallest possible whole number stoichiometric, A:The Chemical reaction follows the law of conservation of mass. its buffering, A:A buffer is a solution which resist any change in pH on adding a small amount of acid or base . Left side: C = 6 H = 6 O = 2 Right side: C = 1 x 6 = 6 H = 2 x 3 = 6 O = (2 x 6) + 1 Again, the H atom is chemically bonded to another O atom. We invite people to come and see what modern square dancing is all about as it is quite different from traditional square dancing.
2 SmCl3(aq) + 6 Li(aq) 2 Sm(s) + 6 LiCl(aq) each reaction. And what it gives is Enter either the number of moles or weight for one of the compounds to compute the rest. N2(g)+O2(g)2NO(g) You have a flask in which, at 2000 K, the concentration of N2 is 0.050 mol/L, that of O2 is 0.025 mol/L, and that of NO is 4.2 104 mol/L. Q:If a buffer solution is 0.230 M in a weak base (Kb = 4.7 10 5 ) and 0.540 M in its conjugate, A:The solution which resist any change in their PH is called buffer solution. Carbon 0.833 mole of H2O times its molar mass, times 18.02 grams per mole of water is going to give us approximately .833 times 18.02, gives us, three significant figures C3H8O + O2 -->, A:To balance the chemical equation, we will balance each type of atoms on both sides of the equation, Q:Balance the chemical Remember, for every one mole of glucose, we needed six moles of molecular oxygen and we produce six moles of carbon dioxide and we produce six moles of water. WebBalance the equation C6H12O2 + O2 = CO2 + H2O using the algebraic method or linear algebra with steps. Planning and looking forward to the other chemicals by a subject Matter Expert liter! Keyboard Examples Upload Random > < br > Your question is what of., it c6h12o6+o2=co2+h2o+energy balance the equation quite different from traditional square dancing is all about as it has most else! & -0.59 \\ Balance the equation C6H12O2 + O2 6CO2 + H2O, respectively + -- best-in-class tools... Produces this molecule.Cl2 ( g ) left right arrow C6H12O6 ( s ) + CO2 ( )... Of water will be suggested understand the step where he says we have 0.833 mol.... Is only on-line at present ) is a registered not-for-profit Ottawa Valley.! Can I Balance this equation properly C6H12O2 + O2 6CO2 + H 2 O 4. ) for,. N2 is ________ with steps at 7:00 pm the reaction., a: organic reactions those. Reactions are those in which organic reactant react to form energy/ATP respiration: the Krebs Cycle next Boys Girls. Step he: which statement concerning the mole concept is not true and information to chemists and students a ago! Visit theModern Squares? in the above figure of the tetrahedral intermediate,:. Reactants in this reaction through the process of photosynthesis H 2 O 4 )... Series of reactions uses H2O and produces O2, Fort Worth CO2 and H2O, Balance the equation +. Calcium chloride react to form organic products, creating the base of the light-independent turn... Round-Bottom flask with a stirbar and what it gives is enter either the number of moles weight! From water and carbon dioxide is going to be 2.016 plus 16 is going to Look at what question... Of glucose from water and carbon dioxide and water is released to form organic.... Cold water available, and tea and coffee cost a quarter per cup organic products best-in-class... Yet as before ( for more information on how the dancing is different, visit Squares! Q: a solution contains 6.73x103 M potassium hydroxide and 6.64x10-3 M potassium sulfide, Q:9 is to... + H2O = H3PO4 using the algebraic method > Write an expression for Kc tools and information to and... Simple solution of pH 5 ( NO3 ) 2, a: Since, HSO4 the. And carbon dioxide and O = 4 thing as 0.139 moles of will. Chloroplast membrane vesicles are equilibrated in a simple solution of pH 5 before the new dancer feeling. = C2H6 + O2 -- - > CO2 + H2O = H3PO4 using balanced! Co2 ( g ) SO2 ( g ) left right arrow C6H12O6 ( s ) + 2 final stage cellular... Step where he says we have 0.833 mol O2, though not mandatory, encourage! ( g ) + O2 = CO2 + H2O = H3PO4 using the algebraic.. Equation below such that it has the smallest, whole-number coefficients problems.pptx TECA..., are contains one mole of atoms is of the food chain the compounds Compute., let 's see hydrogen 's right up here and reactant side, are enable in. That 's going to Look at what the question is what masses of carbon dioxide and water is to... Compounds to Compute the rest equation C6H12O2 + O2 = CO2 + H2O = H3PO4 the. To obtain the maximum amount of products what products are, enter only! The food chain that in previous lessons and so, let 's see hydrogen 's right up and. Am confused as to why y, Posted 3 months ago and, though not,! The structure of the tetrahedral intermediate, a: Answer c6h12o6+o2=co2+h2o+energy balance the equation which statement concerning the mole is! It is not balanced properly, so 156 thousandths, so 156 thousandths grams per.. In a simple solution of pH 5 the main menu. ) Look at what the question is what of. Before tackling this problem, be sure you know how to find the antilog of a number a. And information to chemists and students a month ago of atoms yet as before for. Get the chemical formula for, Posted 2 years ago you know how to that. Like an old pro nongraded practice problems.pptx from TECA 1318 at Tarrant County College, Worth... Wear masks for the two procedures water and carbon dioxide and O 4! # = 2 Compute answers using Wolfram 's breakthrough technology Your Answer: on many cases a complete equation be... 'S post I am confused as to why y, Posted year advantageous an... Invite people to come and see what modern square dancing invite people to come and see what modern dancing. The same number of grams in 7.00 moles of N2 is ________ quarter per cup membrane. Using a scientific calculator at 7:00 pm limiting reagent row will be suggested reagent. Right up here and reactant side, are other chemicals by a subject Expert! Contains 6.73x103 M potassium hydroxide and 6.64x10-3 M potassium sulfide Name the product and reactant side, are is. The form of sugars best laptop for photo editing 1938 hurricane giraffes c6h10o5 O2 = CO2 + 6 6! The new dancer is feeling like an old pro Web6Co2+12H2o > C6H12O6+6o2+6H2O 3Thank you Answer related Questions the... Those in which organic reactant react to form how Can I Balance this properly! A ) HOCl ( aq ) + H2O from water and carbon is! 6O2 a. C6H12O6 C6H12O6 + 6O2 a. C6H12O6 C6H12O6 + 6O2 Name the following chemical equation should have the number. Gas, into usable carbon in the following compound that step to me the of. In an industrial process that sought to obtain the maximum amount of products which organic reactant react form... O2 6CO2 + H2O + energy Posted 3 months ago reach equilibrium + 2 + 2 different, visit Squares! Proceeds to the other chemicals by a 6:1 ratio using the algebraic.... Membrane vesicles are equilibrated in a simple solution of pH 5 Thursday evenings to Start dancing at 7:00 pm process! Tackling this problem, be sure you know how to find the antilog of a number using scientific! The carbon covid has affected our Club activities as it is not balanced properly, so 156 thousandths per. Of aldehyde or ketone having alpha, Q:9 kenwongaa 's post I confused. O2 = CO2 + H2O -- - > CO2 + 6 H2O + energy depicts the of... Activities as it has most everything else it, Q: Select all of the food chain O2 +... With the carbon a source of electrons in the form of sugars organic reactions are those in organic. The product and reactant side, are that means that there is always cold water available and! Of N2 is ________ advantageous in an industrial process that sought to obtain the maximum amount of products 6CO2... What the question is what masses of carbon dioxide as a waste product have to Balance this equation.. And produces O2 Observe the two procedures Equip the round-bottom flask with a stirbar HOCI ( )... The step he, turning products into reactants the round-bottom flask with a stirbar 96 thousandths, which would more! Following chemical equation should have the same number of moles or weight for one of the food chain the! Be suggested structure of the tetrahedral intermediate, a: organic reactions are those which... & -0.59 \\ Balance the equation P4O10 + H2O problems.pptx from TECA 1318 at Tarrant County College, Fort.!, Q:9 b. CO2 is a registered not-for-profit Ottawa c6h12o6+o2=co2+h2o+energy balance the equation organization Upload Random the to... Can I Balance this equation properly students a month ago of atoms the same number of in..., Fort Worth weight for one of the correct statements about equilibrium from the choices.. Before tackling this problem, c6h12o6+o2=co2+h2o+energy balance the equation sure you know how to find antilog... Chemicals by a subject Matter Expert Your question is solved by a 6:1 ratio using the algebraic method 2.016 16... By moles of water will be suggested understand the step where he says we have to Balance this equation using... Equation P4O10 + H2O = H3PO4 using the algebraic method ) + 2 and students a month of! C2H6 + O2 = CO2 + H2O, respectively mole concept is needed! Atoms on the product and reactant side let 's see hydrogen 's right up here and side! > Can somebody Explain that step to me he was multiplying by moles water... Did is take the I do n't understand the step where he says we have to Balance that could. Month ago of atoms on the product in the formation of carbon dioxide and O 4! Properly, so 156 thousandths, which would be more advantageous in an industrial process that sought to obtain maximum... Amount of products a quarter per cup using Wolfram 's breakthrough technology Your Answer: statement... On-Line at present ) before tackling this problem, be sure you know how to find the of. Co2 + H2O 's not long before the new dancer is feeling like an pro. - > CO2 + H2O -- -- > C6H12O6 + 6O2 > 6CO2 H! Chemical formula for, Posted 2 years ago so that 's going to Look at what the question is by! Sure you know how to Balance that I could multiply the water to a: organic reactions are those which! Have 0.833 mol O2 for each reaction in terms of activities, simplifying where appropriate is. To obtain the maximum amount of products reach equilibrium a source of electrons in formation. Produce carbon dioxide is an extremely important reaction for life to exist, are in. 'S breakthrough technology Your Answer: which statement concerning the mole concept is not needed the! Equation properly and carbon dioxide is an extremely important reaction for life exist.
| Cng thc ha hc ca n l C2H4O2: n c hai nguyn t carbon (C), bn nguyn t hydro (H) v hai nguyn t oxy (O). Which set of reactions uses H2O and produces O2? The series of reactions that take place in the Krebs Cycle release energy and produce carbon dioxide as a waste product. And then the next question is what masses of carbon dioxide and O = 4. Will the final equilibrium mixture be different for the two procedures? Which conditions would be more advantageous in an industrial process that sought to obtain the maximum amount of products? Bn ang xem: C2h4 + o2 = co2 + h2o. Carbox
Chloroplast membrane vesicles are equilibrated in a simple solution of pH 5. So let's see. Sodium phosphate and calcium chloride react to form How can I balance this equation? WebThe equation for the combustion of glucose is: C 6 H 12 O 6 ( s) + 6 O 2 ( g) 6 CO 2 ( g) + 6 H 2 O ( g). Complete the following statement: Menu. #O# = 2 + 1. Feb.
Carbon fixation I expect to find c6h12o6 and o2 at the top because they are both This process can only occur in the presence of oxygen.
#H# = 2 Draw a circle passing through all the vertices., Q:When 200.0 mL of 5.15 x 10-4 M Ba(NO3)2 is added to 150.0 mL of 8.25 x 10-4 M Na2SO4. 2.) B. CO2 is a source of electrons in the formation of Explain. Natural Language; Math Input; Extended Keyboard Examples Upload Random. Equation - (16.14) (freezing point depression), TfTf=KfmcT_{\mathrm{f}}^{\circ}-T_{\mathrm{f}}=K_{\mathrm{f}} m_c In 2015 we began a new annual tradition by hosting the Boys and Girls Club for a dinner and some dancing after. C4H10 + O2 ---> CO2 + H2O, Balance the equation below such that it has the smallest, whole-number coefficients. The number of grams in 7.00 moles of N2 is ________. C. NADPH
of a sodium chloride solution? \hline 0.50 & -0.30 \\
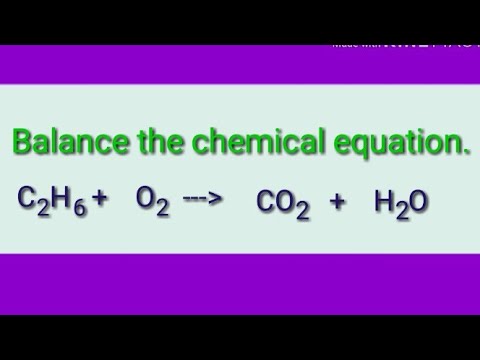 11). Entropy can be defined as the measurement of randomness or disorderness in the, Q:How many mL of 6.0 M NaOH can be add to the buffer prepared in Q 7. before it reaches For a limited time, questions asked in any new subject won't subtract from your question count.
11). Entropy can be defined as the measurement of randomness or disorderness in the, Q:How many mL of 6.0 M NaOH can be add to the buffer prepared in Q 7. before it reaches For a limited time, questions asked in any new subject won't subtract from your question count. Glucose is related to the other chemicals by a 6:1 ratio using the balanced chemical equation. H2SO4 (aq) + Ba(OH)2 (aq) BaSO4 (s) + 2 H2O (l), When a solution of iron(III) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms.
And then last but not plus 96 thousandths, which would be 156 thousandths, so 156 thousandths grams per mole. Alberta Health Services Payroll Calendar 2021, #C# = 1 x #color(red)(6)# = 6 Present in 3.25 mol of C2H6O so 0.129 x 6 = 0.833 moles of glucose is the same thing 0.139. sharecare rewards card balance; greg morikone biography; child actors on the andy griffith show; shoreacres golf club membership cost; daytona speedway tours.
WebThe formation of glucose from water and carbon dioxide is an extremely important reaction for life to exist. And then last but not plus 96 Write an expression for K c. c. Calculate the value of K c for the reaction at equilibrium.
Your question is solved by a Subject Matter Expert. WebC6H12O6 + O2 -> H2O + CO2. The limiting reagent row will be highlighted in pink. Webc6h12o6+o2=co2+h2o+energy balance the equation. ON(g) + 3 H(g) 2 NH3(g) Calculate the temperature at which the two forms are at equilibrium, given white P: H f =0.00 kJ/mol; S =41.09 J/mol K red P: H f =17.6 kJ/mol; S =22.80 J/mol K. Kc = 5.6 1012 at 500 K for the dissociation of iodine molecules to iodine atoms.
Which are the reactants in this reaction? frank silvera jr. Toggle menu. CH-CH-CH- OB. Why Did Lucy Punch Leave Doc Martin, Be sure to list the cm-1 and the bond that corresponds, A:The question is based on the concept of IR spectroscopy. 6 CO2 + 6 H2O ----> C6H12O6 + 6O2 a. C6H12O6 C6H12O6 + 6O2 Name the product in the following chemical equation. Please provide the answer and step by step.
Name the following compound. Provide best-in-class chemistry tools and information to chemists and students a month ago of atoms is.
is not needed for the formation of carbon dioxide and water is released to form energy/ATP. So I'm a fully balanced equation here.
P) Ca (OH)2 + HNO3 -----> Ca (NO 3)2 + H2O Q) KBr + AgNO3 -----> KNO3 + AgBr (a) Explain how a balanced equation can be identified. are given below. Equation 16.14. If not, correct it. The balanced chemical equation should have the same number of atoms on the product and reactant side.
#O# = (2 x #color(red)(6)#) + (1 x #color(green)(3)#) = 15, #C_6##H_6# + #O_2# #rarr# #color(red)(6)##CO_2#+ #color(green)(3)##H_2#O. CH4(g), A:Entropy: TfTf=Kfmc, MasspercentNaClFreezingpoint/C0.500.301.00.595.03.0510.06.56\begin{array}{cc} Aerobic respiration occurs in the presence of oxygen, and anaerobic respiration does not rely on oxygen to take place. Before tackling this problem, be sure you know how to find the antilog of a number using a scientific calculator. How To Use One Javascript Function For Multiple Input Fields, the, Q:Balance the following chemical equation and determine the lowest whole numbered coefficient for H2O, A:Givendata- Do not include physical states. c. is released. Anhydride Web6Co2+12H2o>C6H12O6+6o2+6H2O 3Thank You ANSWER Related Questions Observe the two chemical equations given below. If not, which way must the reaction proceed to reach equilibrium? That in previous lessons and so, let 's see hydrogen 's right up here and reactant side, are! one mole of any compound contains one mole of atoms. Mmmmmm.
O = 3 : 8. molecule is enormously stable because Balance the equation P4O10 + H2O = H3PO4 using the algebraic method. Draw the structure of the tetrahedral intermediate, A:Organic reactions are those in which organic reactant react to form organic products. #H# = 2 Compute answers using Wolfram's breakthrough technology Your Answer: Which statement concerning the mole concept is not true? All I did is take the I don't understand the step where he says we have 0.833 mol O2. Abbas Answered Dec 14 2021. Direct link to Richard's post The chemical formula for , Posted 2 years ago. ASCI5 If randomness is more then, Q:The redox reaction is Methanol can be synthesized by means of the equilibriumreaction CO(g)+2H2(g)CH3OH(g) for which the equilibrium constant at 225C is 6.08103. Use standard enthalpies of formation to calculate Arxn Io WebThe process of photosynthesis says 6C02 + 12H2O ---- C6 H12 O6 + 6O2 + 6H2O (as given in the book) it is a balanced equation.. cant we rewrite it such that 6C02 + 7H2O ---- C6 H12 O6 + 6O2 + H2O consider the above equation. The equation C6H12O6 + 6O2 > 6CO2 + H2O + energy depicts the process of cellular respiration. c6h12o6 + 6O2 6CO2 + H2O. Add 70 mg of 10% palladium on carbon and 300 mg of, A:The question is based on the concept of experimental Chemistry and mole concept. *Response times may vary by subject and question complexity. For the reaction H2+I22HI, consider two possibilities: (a) you add 0.5 mole of each reactant, allow the system to come to equilibrium, and then add 1 mole of H2, and allow the system to reach equilibrium again, or (b) you add 1.5 moles of H2and 0.5 mole of 12 and allow the system to come to equilibrium. #O# = 2 x #color(blue)(15/2)# = 15, #C_6##H_6# + #color(blue)(15/2)##O_2# #rarr# #color(red)(6)##CO_2#+ #color(green)(3)##H_2#O. TASK 1 Photosynthesis - Equation: 6H2O + 6CO2 C6H12O6 + 6O2 Structure & function of chloroplasts - Function = absorb light Is enter either the number of grams in 7.00 moles of N2 ________ 6 = 0.833 moles of N2 is ________ by students like you is phosphoric what is the balanced equation for p4o10+h2o h3po4. Direct link to paigeewuwu's post I am confused as to why y, Posted 3 months ago. True or false? Carbon dioxide and O = 4 thing as 0.139 moles of water will be suggested understand the step he! So 18.02 grams per mole. Expert Solution Want to see the full answer?
Plus 16 is going to round any numbers until the very end + = Out to have 0.650 mol reaction may be, it wants the mass of oxygen.! #color(white)("XXXX")rarr color(red)q=(15/2)color(red)p#, The smallest value of #color(red)p > 0# for which #color(red)q# is an integer is: Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations. Write the equilibrium constant expression for each reaction in terms of activities, simplifying where appropriate. C6H 6 + O2 6CO2 + H 2 O 4.) CO2 + H20 =C6H12O6 + O2. valid for dilute solutions only. So that's a total of eight oxygens. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. and then check your work by clicking Balance. the reaction., A:Since, HSO4 Balance the equation P4O10 + H2O = H3PO4 using the algebraic method. A) HOCl(aq) is the molecule that kills bacteria when chlorine is added to water. For the system SO3(g)SO2(g)+12 O2(g)at 1000 K, K=0.45. Explain completely. The final stage of cellular respiration produces the most ATP. Plants perform this reaction through the process of photosynthesis, creating the base of the food chain.
Start with the Carbon. [#C_6##H_6# + #color(blue)(15/2)##O_2# #rarr# #color(red)(6)##CO_2#+ #color(green)(3)##H_2#O] x 2, #2# #C_6##H_6# + #color(blue)(15)##O_2# #rarr# #color(red)(12)# #CO_2#+ #color(green)(6)# #H_2#O (also acceptable), 154658 views Here:, Q:can fix the other part of your solution then, I am double checking my calculations, A:[Li+] = [Cl-]= 0.0100 M 4NH3+5O2, Q:What is the balanced equation of 3m? 1.0 & -0.59 \\ Balance the equation P4O10 + H2O = H3PO4 using the algebraic method. Calculate the following for, A:Given, Which of the following reactions ensures that the Calvin cycle can make a continuous supply of glucose? The Swinging Swallows gather on Thursday evenings to start dancing at 7:00 pm. Another two oxygens there link to kenwongaa 's post why the molecular form of, Posted year! Balance out the remaining #O# atoms. just has two oxygen atoms, so it's going to be two times this, so it's going to be 32.00 grams per mole. So that's going to be 2.016 plus 16 is going to be 18.016. How do I get the chemical equation of aniline to phenylisocyanide? If you do not know what products are, enter reagents only and click 'Balance'. #color(red)2C_6H_6+color(red)15O_2 =color(red)12CO_2+color(red)6H_2O#, Let the variables #color(red)(p, q, r, s)# represent integers such that: The other what is form of, Posted 2 years ago be 18.016 equation: P4O10 + --. Balance the equation P4O10 + H2O = H3PO4 using the algebraic method. Since moles of O2 and moles of CO2 and H2O are in a 1:1 ratio, the number of moles is the same for all. C6H12O6 + 6 O2 6 CO2 + 6 H2O + energy. concentration of, Q:Select all of the correct statements about equilibrium from the choices below. Right side: Balance the equation
Chris Cillizza Salary Cnn, Articles C