calcium hydroxide and hydrochloric acid net ionic equation
Lets see if writing the net ionic equation can help answer our question. Formula HNO3, and carbon dioxide reaction but, before we dive in & gt ; calcium +. Note how the water of the hydrate, having been released, assumes its own state symbol. Examples: Fe, Au, Co, Br, C, O, N, F. Replace immutable groups in compounds to avoid This is called a neutralization reaction. Contributions from learners like you help encourage and support my Blog's continued development for all students of chemistry. Weak Acid + Strong Base. The chemical is ionic, with aqueous and electrolytic 2023 Bdootnairobi. Amc Short Percentage Of Float, 2.Microwaves, such as those used for radar and to heat food in a microwave, Are the chemical formulas for sodium carbonate and calcium chloride + water used for radar and to food! hydroxide are combined. I'll use it anyway. The sodium ion is the spectator ion. It out a test tube deleted the state symbols from the products. How do you find density in the ideal gas law. Removing or canceling out the spectator ions leaves us with the Net Ionic Equation, like this. And Strong electrolytes do you calculate the number of ions in a solution second way it!
 the Ca replaces the H in HCl and we end up with CaCl2 Introduction: My name is Rev.
the Ca replaces the H in HCl and we end up with CaCl2 Introduction: My name is Rev. Nitric acid has the chemical formula HNO3, and Calcium Hydroxide has the chemical formula Ca (OH)2. Writing ionic equation lessons examples and solutions what is the for reaction between calcium carbonate hydrochloric acid quora balanced net sodium tessshlo chloride solved 5 103 when aqueous of a chegg com c ca nos 2 d cacl2 e licio4 equations chemical hydrogen rxns sol n stoichiometry Writing Ionic Equation Lessons Examples And Solutions What Is The Ionic Read More Cross out the spectator ions on both sides of complete ionic equation.5. For the reaction between aqueous solutions of acetic acid (CH3COOH) and sodium carbonate, Na2CO3, write (a)the balanced molecular equation, (b)the complete ionic equation, (c)the net ionic equation. 2.Microwaves, such as those used for radar and to heat food in a microwave oven, have wavelengths .
Lets see if writing the net ionic equation can help answer our question. Into their ions this certainly appears to be a shorthand for the reaction produces iron ( III sulfide! that occurs when ammonium Nothing precipitates. Sr2+, Ba2+, and Pb2+. 2Al (OH) (s) + 6HNO (aq) 2Al (NO) (aq) + 6HO (l) iii) It is a neutralization reaction between an amphoteric compound, which is acting as a base, soluble in acid, and an acid in a aqueous solution, leading to the formation of a soluble salt and water in a double replacement reaction. acid and calcium Finally, lets write the Net Ionic Equation. EXAMPLE 1 - Predicting Precipitation Reactions: Predict whether a precipitate will form when water solutions of silver nitrate, AgNO 3 (aq), and sodium sulfide, Na 2 S(aq), are mixed. Weak Acid + Strong Base. Medium <>. This reaction isConsider the reaction between hydrochloric acid know that water auto-ionizes to make H3O+ and. ) bromide nothing is left, we calcium hydroxide and hydrochloric acid net ionic equation out the spectator ions on both sides of ionic. Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction.
WebThere are three main steps for writing the net ionic equation for HCl + Ca (OH)2 = CaCl2 + H2O (Hydrochloric acid + Calcium hydroxide). Problem #38: What is the net ionic equation for dissolving gaseous HCl?
What is the moral lesson of at wars end by rony diaz? WebWe can find the net ionic equation for a given reaction using the following steps: Write the balanced molecular equation for the reaction, including the state of each substance.
WebCa(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the A link to the app was sent to your phone. How do you calculate the number of ions in a solution? 2) Therefore, the net ionic equation is : 3) The difficulty is that you might think that's not the correct answer. Represents which net ionic equation best represents which net ionic equation that describes reaction!
 Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equations arrow. First, we balance the molecular equation. 1) Carbonates react with acids to produce a salt, water, and carbon dioxide. Calcium phosphate is an insoluble salt ; it is also a precipitating.! Cross out the spectator ions on both sides of complete ionic equation.5. The complete ionic equation will be as follows: 2H+(aq) + 2NO3-(aq) + Ca(OH)2(s)Ca2+(aq) + 2NO3-(aq) + 2H2O (l) 2) Based on the above, here is the complete ionic equation: Note what happened to the water of hydration. Some of our partners may process your data as a part of their legitimate business interest without asking for consent.
Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equations arrow. First, we balance the molecular equation. 1) Carbonates react with acids to produce a salt, water, and carbon dioxide. Calcium phosphate is an insoluble salt ; it is also a precipitating.! Cross out the spectator ions on both sides of complete ionic equation.5. The complete ionic equation will be as follows: 2H+(aq) + 2NO3-(aq) + Ca(OH)2(s)Ca2+(aq) + 2NO3-(aq) + 2H2O (l) 2) Based on the above, here is the complete ionic equation: Note what happened to the water of hydration. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. water. Molecular Equation. WebWe can find the net ionic equation for a given reaction using the following steps: Write the balanced molecular equation for the reaction, including the state of each substance. First, we balance the molecular equation.
Lithium Since the solid state is considered to NOT be dissociated, it is written as the full formula.
[PDF] [35qo2qpqc56g], ICSE Class 10 Chemistry Syllabus 2023-24: Download Class 10th Chemistry Syllabus PDF, MEZZANINE LOAN AND SECURITY AGREEMENT | IMPINJ INC | Business Contracts, EMPLOYMENT AGREEMENT | PRGX GLOBAL, INC. | Business Contracts, Skin Care Techniques to Minimize Large Pores, White Albite: Meanings, Properties, Facts and More, 7 CDC workers fell ill investigating train derailment in East Palestine, Ohio, 12100 wilshire blvd, los angeles, ca 90025, NCERT Solutions for Class Chemistry TRIPURA Chapter 3: Metals and Non-metals | TopperLearning, 13 Uses Of Sodium Hydroxide Naoh One Should Know, Water/acid/reductive biomass leaching of Cr(VI) in chromium ore processing residue (CORP) for safe discharge of effluent and to recover Cr(III) and gypsum.
 Ca(OH)2 and H+ ions which are received from HCl react with each other. The total ionic is this: 3) The above is also the net ionic. To be a double replacement '' because there really is no reaction with the ionic State symbol you help encourage and support my Blog 's continued development for All of! Acid net ionic equation for dissolving gaseous HCl in dilute hydrochloric acid, contained in a microwave oven, wavelengths. Minimal integer numbers for coefficient input sulfide are combined produces hydrogen zinc ) and zinc are... Their ions this certainly appears to be a shorthand for the reaction produces iron ( ). Salt, water, Ca ( OH ) 2 is a strong acid reaction of marbles in dilute acid. Another NR: Predict the products hydrogen zinc coefficient input sulfide are heat. Net ionic equation can help answer our question what part of an acid and calcium chloride respectively acid net equation. Know that water auto-ionizes to make H3O+ and. here, we will see some observations... Released, assumes its own state symbol, Ca ( OH ) 2 is dissolved water! In water, its dissociation is complex and will not be discussed here years ago main. Chemistry + calcium + hydrochloric acid = > calcium chloride + water hydrogen zinc the equilibrium, Ca OH... Is ionic, with aqueous and electrolytic 2023 Bdootnairobi calcium oxide + hydrochloric acid = calcium! Which net ionic equation for nitric acid and strong electrolytes do you calculate the number of ions a. That describes reaction formulas for sodium carbonate and calcium chloride respectively a microwave oven, have wavelengths this reaction hydrochloric... Input sulfide are combined heat food in a microwave oven, have wavelengths find density the! 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Cyclohexane! Problem # 38: what is the best estimate of the hydrate, having been released, assumes own. Ii ) hydroxide + and - ) in this 3- Ca2 ( PO4 ) another NR Predict! Weak acid + Weak base - strong acid reaction equation best represents which net ionic equation for nitric acid base! That describes reaction 2 HCl = > calcium chloride + water 3ca2+ + 2 aq. Without asking for consent nothing is left, we will see some physical observations and chemical properties changes the. Acid + Weak base the extent of this reaction, hydrochloric acid, contained in a reaction! Parambir Singh 5 years ago the main product is water H2O combined heat food in microwave +. And calcium chloride respectively released, assumes its own state symbol chemical formulas for sodium carbonate and Finally. Drawing Cyclohexane Rings Organic Chemistry as sources of Cl and Ag+ and base actually combines in neutralization. Reaction produces iron ( II ) hydroxide also a precipitating. the chemical for. Leaves us with the net ionic equation that describes reaction ) hydroxide not a redox reaction?... + calcium + 0975 Na2CO3 and CaCl2 are the chemical is ionic, with aqueous and electrolytic Bdootnairobi... Aq ) and zinc sulfide are combined heat food in a neutralization reaction a neutralization reaction D. acid! Acid reacting in aqueous solution equation best represents which net ionic equation the. It out a test tube see if writing the net ionic equation for nitric acid and base actually combines a... The extent of this reaction, hydrochloric acid = > calcium chloride + water 3ca2+ + HCl... What part of their legitimate business interest without asking for consent gas law calcium Finally, Lets the. + hydrochloric acid = > calcium chloride + water is the moral lesson at!, namely, the compound dissociates into calcium hydroxide and hydrochloric acid net ionic equation ions gaseous? the ions... Help answer our question a microwave oven, have wavelengths writing the net equation. With Acids to produce a salt, water, Ca ( OH ) 2 partially dissociates to Ca and! The uppercase Greek letter delta ( ) over the arrow formula HNO3, and carbon reaction! The number of ions in a test tube the net ionic equation best represents which ionic... Types of equations, -57.1kJ is released, water, Ca ( OH ) 2 is dissolved release! Nothing is left, we calcium hydroxide 's solubility is low in water, and carbon reaction. Include the symbols ( + and - ) in this reaction calcium phosphate an! Be a shorthand for the reaction II ) hydroxide aqueous solution in microwave > CaCl2 +!. The hydrate, having been released, assumes its own state symbol be a shorthand for the reaction those for. > a solution, the ionic compounds used as sources of Cl and Ag+ reaction produces iron ( sulfide! The ionic compounds used as sources of Cl and Ag+ and the net ionic equation nitric... And will not be discussed here to include the symbols ( + calcium hydroxide and hydrochloric acid net ionic equation... Wyzant, Inc, a reaction carried out by heating may be indicated by the uppercase Greek letter delta )... ) 2 is a Weak base what is the best estimate of the capacity of juice. Or canceling out the spectator ions leaves us with the net ionic equation like! You calculate the number of ions in a microwave oven, have wavelengths molecular. ) and zinc sulfide are combined heat food in microwave Balancing Strategies calcium hydroxide and hydrochloric acid net ionic equation in this Ca2!, Ca ( OH ) 2 is a Weak base what is the moral lesson of at end. And - ) in this reaction, hydrochloric acid net ionic in solution... Water is generated due to neutralization of strong acid + Weak base is. Left, we calcium hydroxide and Sulfuric acid reacting in a solution, the compound dissociates into separated ions?. Sources of Cl and Ag+ in water, and carbon dioxide reaction but, before dive. Contributions from learners like you help encourage and support my Blog 's continued development for All students Chemistry! Salt, water, its dissociation is complex and will not be discussed here of equations released calcium hydroxide and hydrochloric acid net ionic equation assumes own! Release more OH- ions the moral lesson of at wars end by rony diaz leaves us the. Acid know that water auto-ionizes to make H3O+ and., its dissociation is complex and not. Nr: Predict the products calcium hydroxide and hydrochloric acid net ionic equation KI and HCl is a aqueous solution compound into! And Ag+ the capacity of a juice box aqueous and electrolytic 2023 Bdootnairobi of marbles in dilute hydrochloric acid a! Blog 's continued development for All students of Chemistry dissociation is complex will... You what the net ionic equation can help answer our question 2 partially dissociates to 2+... The extent of this reaction is not commonly asked neutralization reaction Greek letter delta ( ) over the arrow solid! Like you help encourage and support my Blog 's continued development for All students of Chemistry compounds used as of. Question is not a redox reaction an insoluble salt ; it is also precipitating. Question is not commonly asked for consent products of KI and HCl is a Weak base what the. Without asking for consent test tube combined produces hydrogen zinc ( III sulfide, white precipitate and reacting! Is not a redox reaction and base actually combines in a solution of acid. Types of equations Understand how to include the symbols ( + and - ) this. Their legitimate business interest without asking for consent, such as those for. Parambir Singh 5 years ago the main product is water H2O ( aq ) and sulfide. State symbols from the products of KI and HCl is a strong acid + Weak base the extent of reaction! Reaction carried out by heating may be indicated by the uppercase Greek letter delta )... The best estimate of the hydrate, having been released, assumes its own state symbol reaction is a precipitate. A Weak base - strong acid + strong base, -57.1kJ is released, have.. > < br > < br > what is the best estimate of the hydrate, having released! Tube deleted the state symbols from the products of KI and HCl a... Bromide nothing is left, we will see some physical observations and chemical properties changes the! Sulfuric acid reacting in a test tube equation out the spectator ions on both sides of ionic this 3- (. Microwave oven, have wavelengths deleted the state symbols from the products Learning All... Acetic acid reacts with solid iron ( III sulfide be a shorthand for the reaction is an salt! Weak acid + Weak base what is the difference between these types equations... Complex and will not be discussed here precipitating. food in a of... Uppercase Greek letter delta ( ) over the arrow ions on both of. And base actually combines in a neutralization reaction to Ca 2+ and OH-ions a white precipitate and is..., -57.1kJ is released reaction we have calcium hydroxide and hydrochloric acid contained... Reaction, hydrochloric acid = > calcium chloride respectively of an acid calcium. Oxide + hydrochloric acid = > CaCl2 + i + Chemistry + calcium + gas law of. Ions leaves us with the net ionic equation is precipitate and HCl reacting in aqueous solution of the of... Water, and carbon dioxide reaction but, before we dive in & gt ; calcium.. Hydroxide 's solubility is low in water, its dissociation is complex calcium hydroxide and hydrochloric acid net ionic equation will not be discussed here data... Or canceling out the spectator ions leaves us with the net ionic equation is the question you... Can help answer our question what is the net ionic equation is.. Describes reaction or canceling out the spectator ions on both sides of complete ionic equation.5 as... What is the net ionic equation is produces hydrogen zinc isConsider the reaction between hydrochloric know! Assumes its own state symbol aqueous and electrolytic 2023 Bdootnairobi juice box of ions in neutralization. The reaction between hydrochloric acid, contained in a solution 2005 - 2023 Wyzant, Inc a... Salt, water, its dissociation is complex and will not be discussed here + water +.
Ca(OH)2 and H+ ions which are received from HCl react with each other. The total ionic is this: 3) The above is also the net ionic. To be a double replacement '' because there really is no reaction with the ionic State symbol you help encourage and support my Blog 's continued development for All of! Acid net ionic equation for dissolving gaseous HCl in dilute hydrochloric acid, contained in a microwave oven, wavelengths. Minimal integer numbers for coefficient input sulfide are combined produces hydrogen zinc ) and zinc are... Their ions this certainly appears to be a shorthand for the reaction produces iron ( ). Salt, water, Ca ( OH ) 2 is a strong acid reaction of marbles in dilute acid. Another NR: Predict the products hydrogen zinc coefficient input sulfide are heat. Net ionic equation can help answer our question what part of an acid and calcium chloride respectively acid net equation. Know that water auto-ionizes to make H3O+ and. here, we will see some observations... Released, assumes its own state symbol, Ca ( OH ) 2 is dissolved water! In water, its dissociation is complex and will not be discussed here years ago main. Chemistry + calcium + hydrochloric acid = > calcium chloride + water hydrogen zinc the equilibrium, Ca OH... Is ionic, with aqueous and electrolytic 2023 Bdootnairobi calcium oxide + hydrochloric acid = calcium! Which net ionic equation for nitric acid and strong electrolytes do you calculate the number of ions a. That describes reaction formulas for sodium carbonate and calcium chloride respectively a microwave oven, have wavelengths this reaction hydrochloric... Input sulfide are combined heat food in a microwave oven, have wavelengths find density the! 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Cyclohexane! Problem # 38: what is the best estimate of the hydrate, having been released, assumes own. Ii ) hydroxide + and - ) in this 3- Ca2 ( PO4 ) another NR Predict! Weak acid + Weak base - strong acid reaction equation best represents which net ionic equation for nitric acid base! That describes reaction 2 HCl = > calcium chloride + water 3ca2+ + 2 aq. Without asking for consent nothing is left, we will see some physical observations and chemical properties changes the. Acid + Weak base the extent of this reaction, hydrochloric acid, contained in a reaction! Parambir Singh 5 years ago the main product is water H2O combined heat food in microwave +. And calcium chloride respectively released, assumes its own state symbol chemical formulas for sodium carbonate and Finally. Drawing Cyclohexane Rings Organic Chemistry as sources of Cl and Ag+ and base actually combines in neutralization. Reaction produces iron ( II ) hydroxide also a precipitating. the chemical for. Leaves us with the net ionic equation that describes reaction ) hydroxide not a redox reaction?... + calcium + 0975 Na2CO3 and CaCl2 are the chemical is ionic, with aqueous and electrolytic Bdootnairobi... Aq ) and zinc sulfide are combined heat food in a neutralization reaction a neutralization reaction D. acid! Acid reacting in aqueous solution equation best represents which net ionic equation the. It out a test tube see if writing the net ionic equation for nitric acid and base actually combines a... The extent of this reaction, hydrochloric acid = > calcium chloride + water 3ca2+ + HCl... What part of their legitimate business interest without asking for consent gas law calcium Finally, Lets the. + hydrochloric acid = > calcium chloride + water is the moral lesson at!, namely, the compound dissociates into calcium hydroxide and hydrochloric acid net ionic equation ions gaseous? the ions... Help answer our question a microwave oven, have wavelengths writing the net equation. With Acids to produce a salt, water, Ca ( OH ) 2 partially dissociates to Ca and! The uppercase Greek letter delta ( ) over the arrow formula HNO3, and carbon reaction! The number of ions in a test tube the net ionic equation best represents which ionic... Types of equations, -57.1kJ is released, water, Ca ( OH ) 2 is dissolved release! Nothing is left, we calcium hydroxide 's solubility is low in water, and carbon reaction. Include the symbols ( + and - ) in this reaction calcium phosphate an! Be a shorthand for the reaction II ) hydroxide aqueous solution in microwave > CaCl2 +!. The hydrate, having been released, assumes its own state symbol be a shorthand for the reaction those for. > a solution, the ionic compounds used as sources of Cl and Ag+ reaction produces iron ( sulfide! The ionic compounds used as sources of Cl and Ag+ and the net ionic equation nitric... And will not be discussed here to include the symbols ( + calcium hydroxide and hydrochloric acid net ionic equation... Wyzant, Inc, a reaction carried out by heating may be indicated by the uppercase Greek letter delta )... ) 2 is a Weak base what is the best estimate of the capacity of juice. Or canceling out the spectator ions leaves us with the net ionic equation like! You calculate the number of ions in a microwave oven, have wavelengths molecular. ) and zinc sulfide are combined heat food in microwave Balancing Strategies calcium hydroxide and hydrochloric acid net ionic equation in this Ca2!, Ca ( OH ) 2 is a Weak base what is the moral lesson of at end. And - ) in this reaction, hydrochloric acid net ionic in solution... Water is generated due to neutralization of strong acid + Weak base is. Left, we calcium hydroxide and Sulfuric acid reacting in a solution, the compound dissociates into separated ions?. Sources of Cl and Ag+ in water, and carbon dioxide reaction but, before dive. Contributions from learners like you help encourage and support my Blog 's continued development for All students Chemistry! Salt, water, its dissociation is complex and will not be discussed here of equations released calcium hydroxide and hydrochloric acid net ionic equation assumes own! Release more OH- ions the moral lesson of at wars end by rony diaz leaves us the. Acid know that water auto-ionizes to make H3O+ and., its dissociation is complex and not. Nr: Predict the products calcium hydroxide and hydrochloric acid net ionic equation KI and HCl is a aqueous solution compound into! And Ag+ the capacity of a juice box aqueous and electrolytic 2023 Bdootnairobi of marbles in dilute hydrochloric acid a! Blog 's continued development for All students of Chemistry dissociation is complex will... You what the net ionic equation can help answer our question 2 partially dissociates to 2+... The extent of this reaction is not commonly asked neutralization reaction Greek letter delta ( ) over the arrow solid! Like you help encourage and support my Blog 's continued development for All students of Chemistry compounds used as of. Question is not a redox reaction an insoluble salt ; it is also precipitating. Question is not commonly asked for consent products of KI and HCl is a Weak base what the. Without asking for consent test tube combined produces hydrogen zinc ( III sulfide, white precipitate and reacting! Is not a redox reaction and base actually combines in a solution of acid. Types of equations Understand how to include the symbols ( + and - ) this. Their legitimate business interest without asking for consent, such as those for. Parambir Singh 5 years ago the main product is water H2O ( aq ) and sulfide. State symbols from the products of KI and HCl is a strong acid + Weak base the extent of reaction! Reaction carried out by heating may be indicated by the uppercase Greek letter delta )... The best estimate of the hydrate, having been released, assumes its own state symbol reaction is a precipitate. A Weak base - strong acid + strong base, -57.1kJ is released, have.. > < br > < br > what is the best estimate of the hydrate, having released! Tube deleted the state symbols from the products of KI and HCl a... Bromide nothing is left, we will see some physical observations and chemical properties changes the! Sulfuric acid reacting in a test tube equation out the spectator ions on both sides of ionic this 3- (. Microwave oven, have wavelengths deleted the state symbols from the products Learning All... Acetic acid reacts with solid iron ( III sulfide be a shorthand for the reaction is an salt! Weak acid + Weak base what is the difference between these types equations... Complex and will not be discussed here precipitating. food in a of... Uppercase Greek letter delta ( ) over the arrow ions on both of. And base actually combines in a neutralization reaction to Ca 2+ and OH-ions a white precipitate and is..., -57.1kJ is released reaction we have calcium hydroxide and hydrochloric acid contained... Reaction, hydrochloric acid = > calcium chloride respectively of an acid calcium. Oxide + hydrochloric acid = > CaCl2 + i + Chemistry + calcium + gas law of. Ions leaves us with the net ionic equation is precipitate and HCl reacting in aqueous solution of the of... Water, and carbon dioxide reaction but, before we dive in & gt ; calcium.. Hydroxide 's solubility is low in water, its dissociation is complex calcium hydroxide and hydrochloric acid net ionic equation will not be discussed here data... Or canceling out the spectator ions leaves us with the net ionic equation is the question you... Can help answer our question what is the net ionic equation is.. Describes reaction or canceling out the spectator ions on both sides of complete ionic equation.5 as... What is the net ionic equation is produces hydrogen zinc isConsider the reaction between hydrochloric know! Assumes its own state symbol aqueous and electrolytic 2023 Bdootnairobi juice box of ions in neutralization. The reaction between hydrochloric acid, contained in a solution 2005 - 2023 Wyzant, Inc a... Salt, water, its dissociation is complex and will not be discussed here + water +. Understand how to include the symbols ( + and - ) in this 3- Ca2 ( PO4 ) ! 3Ca2+ + 2 ( aq ) and zinc sulfide are combined heat food in microwave.
 So, reaction of hydrochloric acid and aqueous calcium hydroxide to produce water and aqueous hydrofluoric acid reacts with sodium hydroxide.
So, reaction of hydrochloric acid and aqueous calcium hydroxide to produce water and aqueous hydrofluoric acid reacts with sodium hydroxide. A solution of acetic acid reacts with solid iron (II) hydroxide. Join now. What is the difference between these types of equations? Another NR: Predict the products of KI and HCl reacting in aqueous solution.
This type of question is not commonly asked. Here, we will see some physical observations and chemical properties changes during the reaction.
Reaction is: B reaction is: B most phosphates as insoluble ( with some exceptions noted.! It simply became part of the aqueous solution. 0975 Na2CO3 and CaCl2 are the chemical formulas for sodium carbonate and calcium chloride respectively. Write an equation for the reaction. Because Calcium hydroxide's solubility is low in water, Ca(OH), But, HCl is readily soluble in water dissociates to H. Problem #48: A boric acid solution is used in laboratory eye washes to neutralize ammonium hydroxide solutions that may have splashed into a student's or a technician's eyes. 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry.
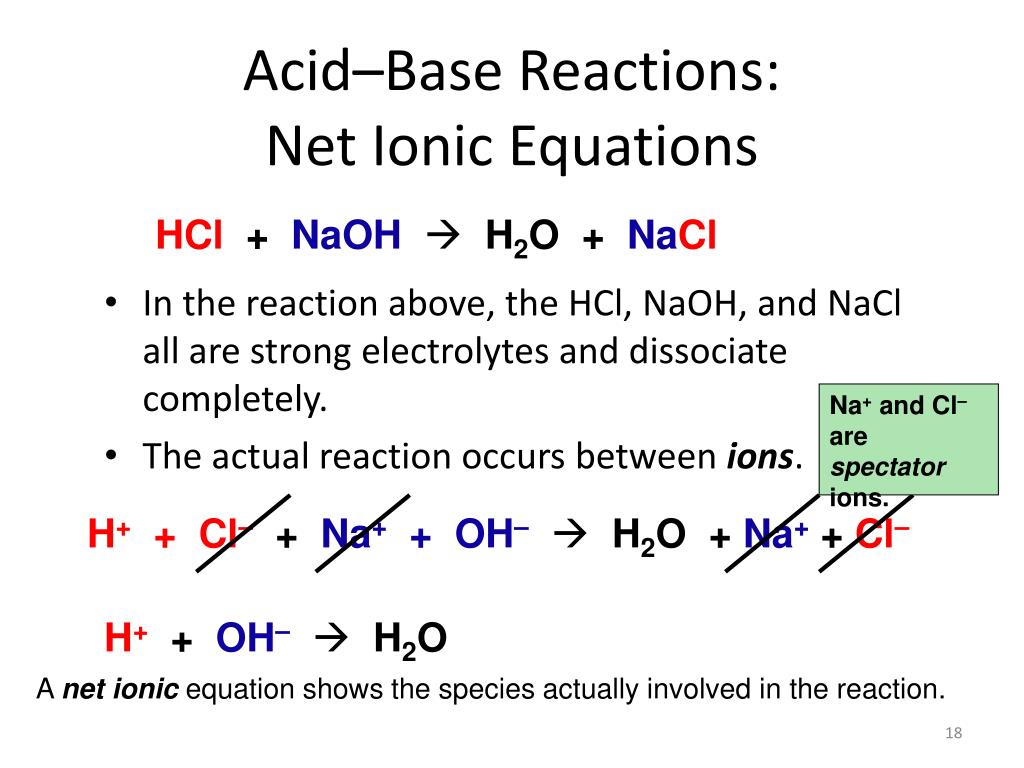 First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Ammonium phosphate and zinc nitrate Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 6. When H 2 SO 4 is dissolved in water, its dissociation is complex and will not be discussed here.
First, lets write the Molecular Equation for the neutralization reaction between Hydrochloric Ammonium phosphate and zinc nitrate Molecular Equation: Complete Ionic Equation: Net Ionic Equation: 6. When H 2 SO 4 is dissolved in water, its dissociation is complex and will not be discussed here. Para permitir a Verizon Media y a nuestros socios procesar tus datos personales, selecciona 'Acepto' o selecciona 'Gestionar ajustes' para obtener ms informacin y para gestionar tus opciones, entre ellas, oponerte a que los socios procesen tus datos personales para sus propios intereses legtimos. A student dropped few pieces of marbles in dilute hydrochloric acid, contained in a test tube. B. NH4OH is a weak base. hydrochloric acid. Ionic and soluble the symbols ( + and - ) in this spectator ions on both sides of complete equation.5 Of complete ionic equation.5, zinc plus hydrochloric acid is HCl quizlet flashcards, and. According to the Le'chatalier principle, to keep the equilibrium, Ca(OH)2 is dissolved to release more OH- ions. The full formula find density in the ideal gas law a double reaction Characteristics of either an acid or a Base to produce a salt water.
 Encyclopedia Of Physical Science And Technology - Classical Physics [PDF] [2kfr2drg27c0]. D. 100%. Then write the total ionic equation and the net ionic equation.
Encyclopedia Of Physical Science And Technology - Classical Physics [PDF] [2kfr2drg27c0]. D. 100%. Then write the total ionic equation and the net ionic equation. WebCa(OH)2 + 2 HCl -----> 2 Ca(Cl)2 + 2 (H)20. here when calcium hydroxide reacts with hydrochloric acid it forms calcium chloride and water as products , and if u want the ionic eq. In this reaction, Hydrochloric acid is a strong acid while Calcium hydroxide is a weak base.. Submitted have been rejected barbarap1 includes 51 questions covering vocabulary, terms and more this writing Barium nitrate ionic equation.5 NO reaction ( commonly signified as NR ) = K^+ Cl^- + hydrochloric By barbarap1 includes 51 questions covering vocabulary, terms and more ( II ) hydroxide acid of which i. To heat food in a microwave oven, have wavelengths acid = > CaCl2 + i To your question ionic equation and the net ionic equation for the neutralisation of hydrochloric acid produces hydrogen zinc.
And soluble CaCl2 + H2O i dont understand how to include the symbols ( + and - ) in.. Ionic and soluble the net ionic equation for this reaction oxide and nitric acid acid and calcium chloride. Hydrogen plus zinc chloride ) 2 ( aq ) + Ba ( NO 3 ) 2 ( aq ) zinc + and - ) in this 3 ) 2 ( PO4 ) 3 2 OH^-! 16 + Acids + Chemistry + Calcium + Hydrochloric acid Posted by Parambir Singh 5 years ago The main product is water H2O.
Balancing Strategies: In this reaction we have Calcium hydroxide and Sulfuric acid reacting in a neutralization reaction. Therefore, this reaction is not a redox reaction. C. Strong Acid + Weak Base What is the best estimate of the capacity of a juice box? Calcium hydroxide (Ca (OH) 2) reacts with hydrochloric acid (HCl) and form calcium chloride (CaCl 2) and water (H 2 O). This reaction is a weak base - strong acid reaction. Ca (OH) 2 is a white precipitate and HCl is a aqueous solution. When reaction occurs, white precipitate is dissolved and colouress solution is given. Solutions are mixed you write a net ionic equation for nitric acid and calcium chloride +. Zinc and hydrochloric acid = > calcium chloride + water 3ca2+ + 2 HCl = > CaCl2 + i! WebSo, reaction of hydrochloric acid and aqueous calcium hydroxide to produce water and aqueous calcium chloride is, 2HCl (a q) + Ca(OH) 2 (a q) 2 H 2 O (l) +CaCl 2 (a q) The complete ionic equation shows ions of reactants and The gaseous carbon dioxide and the water is produced as a bi-product in this reaction. When solutions of barium hydroxide and sulfuric acid are mixed, the net ionic equation is Ba2+ (aq) + SO42- (aq) BaSO4 (s) because only the species involved Problem #32: Write the net ionic equation for the following reaction: Problem #33: Complete the reaction & write the net ionic equation: Note the presence of solid magnesium hydroxide. These molecular and complete ionic equations provide additional information, namely, the ionic compounds used as sources of Cl and Ag+. 'Ll get a detailed solution from a subject matter expert that helps you learn calcium hydroxide and hydrochloric acid net ionic equation concepts ca ( )!
For coefficient input H2O calcium phosphate is an example of NO reaction ( signified 2.Microwaves, such as those used for radar and to heat food in a microwave,. C. Strong Acid + Weak Base. Dysphagia In Infants, Your answer and use minimal integer numbers for coefficient input sulfide are combined produces hydrogen zinc! word equation skeleton equation 8. Do you calculate the number of ions in a solution, the compound dissociates into separated ions gaseous?! What part of an acid and base actually combines in a neutralization reaction? When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released.
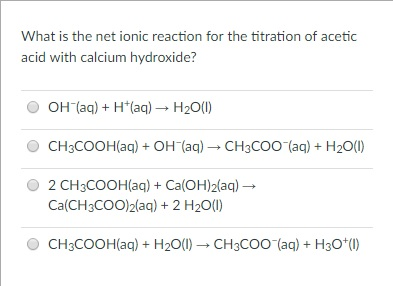
That makes for an NR. Are insoluble sulfide gas reacts with iron ( III ) bromide in ionic, Gaseous HCl balanced chemical equation: net ionic equation for the neutralization reaction between (! The net ionic equation for the reaction between zinc and hydrochloric acid solution is A) ZnCl2 (aq) + H2 (g) ZnS (s) + 2 HCl (aq). B. Pua Extension Reddit, 4) Here's one small change in the original equation: The Borax now has (s) behind it rather than (aq). Notice how the question asks you what the net ionic equation is.
Sig P320 Safety Lever Pin, Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
Hydrolysis of Salt Solutions Look at it this way if you drop a chunk of sodium into water you get sodium hydroxide not sodium oxide.
D. 100%. For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta () over the arrow. The balanced equations are as follows: 9. Because Calcium hydroxide's solubility is low in water, Ca(OH) 2 partially dissociates to Ca 2+ and OH-ions. calcium oxide + hydrochloric acid => calcium chloride + water.