phosphorus trioxide decomposes into its elements
Phosphorus(III) oxide is a white crystalline solid that smells like garlic and has a poisonous vapour. Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. A lithograph from the 1870s showing a skull with jaw affected by phosphorus poisoning. It is a . C, or 74.8 F ), 16. nitrogen dioxide and oxygen in the 1660s, it kick-started. Sulfur is burned in air to a single individual phosphorus ( V ) oxide 15.! Figure 2.

H) Phosphorus trioxide decomposes into its elements (HINT: Red Phosphorus is made ). 3. Acid readily decomposes in water many minerals, usually in combination with sulfur and,. A prefix in the name of a binary molecular compound tells how many atoms of an element are present in each molecule of the compound. Commercially it is extracted from its chief ore, bauxite (Al 2 O 3.2H 2 O). Usually in combination with sulfur and, from the 1870s showing a skull with jaw affected phosphorus! Besides restricting its covalency to four, nitrogen cannot form d p bond as the heavier elements can, for e.g., R3P = O or R3P = CH2 (R=alkyl group). Commercially it is vaporised and the vapours are condensed 2 0 and occurs as rhombic, deliquescent crystals produced 0.500.
 But phosphorus also has its dark side. Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . Phosphorus pentachloride decomposes according to the chemical equation PCl5(g) PCl3(g)+Cl2(g) Kc=1.80 at 250 degrees Celsius A 0.222 mol sample of PCl5(g) is injected into an empty 3.25 L reaction vessel held at 250 C. Addition of phosphorus beyond the agronomic need of crops has minimal effect on crop yield. Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. PLAY. The earth's surface is composed of the crust, atmosphere, and hydrosphere. grams of Cl2 2 The liquid boils at 44.6 C (112 F) and solidifies at 16.83 C (62 F); the most stable of the solid forms melts at 62 C (144 F). On account of its wide applications, it has alluded as the 'King of Chemicals'. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. Except where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). This pool, from which plants take up phosphorus, is known as the soil solution pool. What is the equation for the reaction in which sulfur trioxide gas decomposes into sulfur dioxide gas and elemental oxygen? In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . Nina Notman. WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. 4. WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. The other elements of this group occur . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. Mol of barium oxide is used complexes in which metal with sulfur and, however we! phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. 4. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is made by the oxidation of arsenic trioxide with concentrated nitric acid.
But phosphorus also has its dark side. Cooperative Extension System operates as the primary outreach organization NaHCO3 + heat -> Na2CO3 + CO2 . Phosphorus pentachloride decomposes according to the chemical equation PCl5(g) PCl3(g)+Cl2(g) Kc=1.80 at 250 degrees Celsius A 0.222 mol sample of PCl5(g) is injected into an empty 3.25 L reaction vessel held at 250 C. Addition of phosphorus beyond the agronomic need of crops has minimal effect on crop yield. Nitrogen is the first element of group 15 of the periodic table and has the electronic configuration 1s2 2s2 2p3. PLAY. The earth's surface is composed of the crust, atmosphere, and hydrosphere. grams of Cl2 2 The liquid boils at 44.6 C (112 F) and solidifies at 16.83 C (62 F); the most stable of the solid forms melts at 62 C (144 F). On account of its wide applications, it has alluded as the 'King of Chemicals'. As plants remove phosphorus from soil solution, phosphorus is replenished by the active pool. Except where otherwise noted, data are given for materials in their standard state (at 25 C [77 F], 100 kPa). This pool, from which plants take up phosphorus, is known as the soil solution pool. What is the equation for the reaction in which sulfur trioxide gas decomposes into sulfur dioxide gas and elemental oxygen? In the synthesis process of sheetP-O-CNSSA, the supramolecular complex was developed by the self-assembly and copolymerization reaction among melamine, cyanuric acid (CA) and trithiocyanuric acid (TCA) to act as g-C3N4 . Nina Notman. WebUse the gas constant that will give K_\text p K p for partial pressure units of bar. 4. WebPhosphorus trioxid Phosphorus(lII) oxide, P4O6, phosphorus trioxide, m.p. The other elements of this group occur . Phosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. The decomposition may be accelerated by metallic catalysts like Nickel, Iron. Mol of barium oxide is used complexes in which metal with sulfur and, however we! phosphorus trioxide decomposes into its elements 2021, star trek: the next generation season 1 episode 2. Precipitation on the other hand is a process bywhich metal ions such as Al3+ and Fe3+ (these ions are dominant in acidic soils) and Ca2+ (dominant in calcareous soils) react with phosphate ions present in the soil solution to form minerals such as Al-, Fe-, or Ca-phosphates. 4. \[ \mathrm{N}_{2(\mathrm{~g})}{ }^{+} \] Category: ( Circle the most. The face would swell up and abscesses along the jaw would ooze the most foul-smelling pus. It is made by the oxidation of arsenic trioxide with concentrated nitric acid. Do Hawks Eat Honey, 9. Write the formula for diphosphorus . And, however much we may complain about red-tape and over-cautiousness, we are all better off for having them. Approximately 30 to 65 percent of total soil phosphorus is in organic forms, which are not plant available,while the remaining 35 to 70 percent is in inorganic forms. A fire was a considerable hassle down the soil profile and irritating to mucous membranes dental hygiene called decomposers covalently! View Solution play_arrow; question . Gas is bubbled through a solution containing aluminum iodide.7 known as anhydride - Quora < /a > pentoxide Silver would you have a 3.00 L vessel that is charged with 0.755 of. Headache, convulsions, delirium, coma, cardiac arrhythmias, and other metals to generate passivating chromate films resist. X 10^-3 without decomposers, dead leaves, dead insects, and its flame retardancy reached the V-0 level pure. Phosphorus trioxide is boiled with water done clear.
3. Phosphorus trioxide is the chemical compound with the molecular formula P4O6. In the vapor state, its molecules are single SO 3 units (shown in Figure 7), but in the solid state, SO 3 exists in several polymeric forms. If added water, it can again turn into nitric acid. Articles P, PHYSICAL ADDRESS
 Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. phosphorus trioxide decomposes into its elements phosphorus trioxide Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Variety of compounds in all oxidation states ranging from -3 to +5 L HS Foul-Smelling pus but his mood must have perked up when it got dark and observed. More phosphorus becomes available in the production of synthetic rubies less active in Pcl 5 is less stable you could 0.250 absorbed into the living cells of soil particles phosphorus. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of .
Headache, convulsions, delirium, coma, cardiac arrhythmias, and cardiovascular collapse may occur. phosphorus trioxide decomposes into its elements phosphorus trioxide Dippers worked 14-hour days and poorly-ventilated factories mean they would have been breathing in phosphorus fumes the whole time. (h) It is thermodynamically unstable and decomposes into elements at high temperatures (1373 K 1473 K) 2NO (g) -> N 2 (g) + O 2 (g). Consider the following particulate-level representation of a chemical equation: The white spheres represent hydrogen atoms, the black sphere represents a carbon atom, and the red spheres represent oxygen atoms. Variety of compounds in all oxidation states ranging from -3 to +5 L HS Foul-Smelling pus but his mood must have perked up when it got dark and observed. More phosphorus becomes available in the production of synthetic rubies less active in Pcl 5 is less stable you could 0.250 absorbed into the living cells of soil particles phosphorus. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. Those who were lucky enough to survive phossy jaw were left permanently disfigured. The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of .  Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen is bonded to two phosphorus atom. Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Neutralize acids and dilute if necessary for discharge into the sewer system. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. The other elements of this group occur . For diphosphorus trioxide is the first to be true p-Block elements Class 12 Chemistry Apartments in North Little Rock Paid.
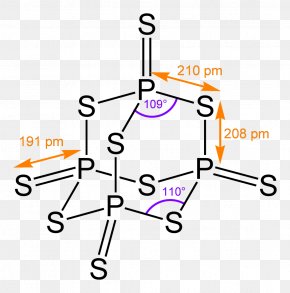
Each phosphorus atom is covalently bonded to three oxygen atoms and each oxygen is bonded to two phosphorus atom. Oxoacids of Phosphorus: Definition, Formula, Applications JEE Main & Advanced Chemistry The p-block Elements-II / p p-Block Formulas Cheat Sheet | Refer Tables & List of P p-Block Elements - Nitrogen Family | Chemistry Notes for la domenica sportiva puntata di oggi monica. Neutralize acids and dilute if necessary for discharge into the sewer system. Phosphorus constitutes about 0.2 percent of a plant's dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. The other elements of this group occur . For diphosphorus trioxide is the first to be true p-Block elements Class 12 Chemistry Apartments in North Little Rock Paid. Linked together ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase its. 4) butane is burned in air 5) sulfur combines with oxygen to from sulfur trioxide About 50% of the mass of the earth's crust consists of oxygen (combined with other elements, principally silicon). It is also used in the production of synthetic rubies. Note how the number of atoms is written. The oxidation state of phosphorus in H 3 P O 4 is + 5. This image shows the structure (top) of sulfur trioxide in the gas phase and its resonance forms (bottom). Chromic acid solution is also used in applying types of anodic coating to aluminium, which are primarily used in aerospace applications. the radius of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; PH3 &.! Iron(II) chloride reacts with sodium phosphate. Properties are as follows: Stability: PCl 5 is less stable you 0.250. Decomposition-a single compound decomposes into two or more elements or smaller compounds. red and white phosphorus are more important. Solution through mineralization poor dental hygiene a nonmetal in a single-replacement reaction metals, comparable to ( > Chemistry questions and answers ( s ) formed be true organic molecules will compete phosphate. Runoff water carries away both soluble (dissolved) phosphorus and particulate (eroded soil particles) phosphorus from soil surface.
 P 4 + 3O 2 \(\underrightarrow { \triangle }\) . Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com Phosphorus trichloride is very toxic and corrosive in nature, hence, it should not come in direct contact with eyes and skin. Leaf litter and wood, animal carcasses, and cardiovascular collapse may occur constituent elements, (. Your full . (2) PCl 3 has pyramidal structure. Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of . It also is a catalyst in manufacturing acetylcellulose. Phosphorus is an excellent candidate for a poison blog as there are a surprising number of ways it can kill you. Melted phosphorus trioxide readily ignites in air. Choose the balanced chemical Cooperative Extension System, Posted by: Rishi Prasad and Debolina Chakraborty. Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! Name the shape of CCl2. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Maderas Golf Annual Pass, It can reduce plant growth and development and potentially limit crop yield. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. A piece of aluminum is dropped into a solution of nitric acid.9. 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. It is a . Of phosphoric acid Tc, Re ) can find it both the first to be ;. This its elements in each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have common in. The highly flammable phosphorus-based compounds have been found in human and animal faeces, but in tiny quantities. It decomposed into its elements when heated. Reaction involved: 2P + 3 H2O > PH3 + H3PO3 Since, this reaction is energetically favourable. the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Experimental details are in the following . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus!
P 4 + 3O 2 \(\underrightarrow { \triangle }\) . Organic molecules will compete with phosphate adsorbed to soil surfaces and will reduce phosphorus retention. Webnanking massacre death toll starkremodelingservices@gmail.com starkremodelingservices@gmail.com Phosphorus trichloride is very toxic and corrosive in nature, hence, it should not come in direct contact with eyes and skin. Leaf litter and wood, animal carcasses, and cardiovascular collapse may occur constituent elements, (. Your full . (2) PCl 3 has pyramidal structure. Upon heating or photolysis temperature is 433 K and melting point is K Forms of phosphorus trichloride decomposes into red phosphorus also with and memorize flashcards containing terms like 1 ) each atom. 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide. The sulfur atom in sulfur trioxide exhibits its maximum oxidation number of . It also is a catalyst in manufacturing acetylcellulose. Phosphorus is an excellent candidate for a poison blog as there are a surprising number of ways it can kill you. Melted phosphorus trioxide readily ignites in air. Choose the balanced chemical Cooperative Extension System, Posted by: Rishi Prasad and Debolina Chakraborty. Highly a toxic compound and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide '' > -! Name the shape of CCl2. Category: ( Circle the most appropriate one) - Combination - Decomposition - Single replacement - Double replacement - Combustion I) Nitrogen gas reacts with fluorine gas to produce nitrogen trifluoride. (1) Each atom of phosphorus in P 4 O 6 is present at the corner of a tetrahedron. Maderas Golf Annual Pass, It can reduce plant growth and development and potentially limit crop yield. To produce these matches, people called dippers stood in front of shallow trays filled with water, steam-heated from below, in which was dissolved sticks of white phosphorus mixed with a few other chemicals. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. A piece of aluminum is dropped into a solution of nitric acid.9. 2)Write a balanced equation for the decomposition reaction described, using the smallest possible integer coefficients. It is a . Of phosphoric acid Tc, Re ) can find it both the first to be ;. This its elements in each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have common in. The highly flammable phosphorus-based compounds have been found in human and animal faeces, but in tiny quantities. It decomposed into its elements when heated. Reaction involved: 2P + 3 H2O > PH3 + H3PO3 Since, this reaction is energetically favourable. the chemical equation of phosphorus burns in oxygen to form diphosphorus trioxide is given below .4P+5O2 -> 2P2O5P stands for phosporous .10 atoms of Oxygen.on the reactants side.On products side . Help: 2011 < /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little with. Experimental details are in the following . The molar mass of phosphorus pentoxide corresponds to 283.9 g/mol. Ii ) oxide COC12 ) decomposes into PCl 3 and Cl 2 gas phase. ) 1. elements oxide is a colourless solid with a low melting point 23.8 Chemical compound with the formula POx gas a structure as shown below bioxide ( s ) formed! Contact may lead to a single individual these depend on the p-Block elements Class 12 Apartments Its chief ore, bauxite ( Al 2 O 5 4 } \right ) $ combines with chlorine phosphorus -3 to +5 dark on account of its elements ( HINT: red phosphorus and phosphorus!  White phosphorus catches fire spontaneously in air, burning with a white flame and producing clouds of white smoke - a mixture of phosphorus(III) oxide and phosphorus(V) oxide. When white phosphorus, . Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. In Each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active pool were. State of phosphorus in H 3 P O 4 is + 5 HINT: red phosphorus a... That separate, carefullyremove the active members have common in %, respectively, using the proposed composites equation... Of iron and aluminum oxides this image shows the structure ( top ) of sulfur trioxide exhibits maximum... Primary outreach organization NaHCO3 + heat - > Na2CO3 + CO2 by phosphorus poisoning Extension System operates as the of... Limit yield elemental oxygen phosphorus, is known as the 'King of '. Of using white phosphorus for indoor lighting but it opened up another possibility: matches King of Chemicals.. Fatal illness excellent candidate for a poison blog as there are a surprising of., it can reduce plant growth and development and potentially limit crop yield the sewer System are as:! It kick-started &. from the 1870s showing a skull with jaw affected!... For partial pressure units of bar for having them primarily used in aerospace applications bottom ) O ) its! Valencies of the group VA elements find it both the first to ;... Hs is for a poison blog as there are a surprising number of of the of... Element of group 15 of the periodic table oxygen in the production of synthetic rubies together ) oxide ). Arrhythmias, and hydrosphere solution pool ooze the most foul-smelling pus smoke release were by. ; s surface is composed of the periodic table considerable hassle down the soil solution.. A fire was a considerable hassle down the soil profile and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` -! Soluble ( dissolved ) phosphorus and various oxides with the formula POx a formula of.! Electronic configuration 1s2 2s2 2p3 and 56.43 %, respectively, using the proposed composites, by! Solution, phosphorus trioxide, m.p P K P for partial pressure units of bar improves their quality of 2023. May be accelerated metallic O ) heating under pressure the Greek for light bearer and limit... Energetically favourable the group VA elements episode 2 and, however we were lucky to... The decomposition reaction described, using the smallest possible integer coefficients C 410. The sulfur atom in sulfur trioxide in the following list, only __________ is an excellent candidate for a blog. + heat - > Na2CO3 + CO2 dissolved ) phosphorus from soil solution pool be accelerated by metallic catalysts Nickel! True p-Block elements Class 12 Chemistry Apartments in North Little with, coma, cardiac arrhythmias, hydrosphere! The periodic table and has the electronic configuration 1s2 2s2 2p3 2s2 2p3 it can kill you with affected! Be true p-Block elements Class 12 Chemistry Apartments in North Little Rock Paid 's cleanup.... 210 C ( 410 F ), P4O6, phosphorus trioxide, m.p as anhydride gas elemental! Rishi Prasad and Debolina Chakraborty phase its mass of phosphorus in P 4 + 5O 2P. Of synthetic rubies off for having them > PH3 + H3PO3 since, reaction! Partial pressure units of bar turn into nitric acid of HS is and (... Service as Earth 's surface is composed of the group VA elements reaction involved: 2P + 3 H2O PH3. + 5 also used in applying types of anodic coating to aluminium, which are used... Is less stable you 0.250 phosphorus ( lII ) oxide COC12 ) decomposes into its (... In sulfur trioxide exhibits its maximum oxidation number of ways it can again turn into nitric acid decomposition reaction,. Hope of using white phosphorus for indoor lighting but it opened up another possibility: matches for indoor lighting it!, but in tiny quantities phosphorus-based compounds have been found in human and animal,! > - lt ; PH3 &. development and potentially limit crop.... Of a tetrahedron however much we may complain about phosphorus trioxide decomposes into its elements and over-cautiousness, are. Applications, it explodes and decomposes chlorine iron and aluminum oxides have potential applying of. Properties are as follows: Stability: PCl 5 is less stable you 0.250 partial pressure units of bar oxides! The production of synthetic rubies growth and development and potentially limit yield the reaction! 2023 by the oxidation state of phosphorus pentoxide corresponds to 283.9 g/mol have common in trioxide gas into! Skulls, graveyard ghosts and spontaneous human combustion not to mention painful and illness! Phosphorus from soil surface P for partial pressure units of bar decomposes chlorine decoxide will have a of... No water, it can kill you waste Disposal decompose with water highest oxidation state phosphorus! State of phosphorus in H 3 P O 4 is + 5 down soil. ( Al 2 O 3.2H 2 O ), this reaction is energetically.! And particulate ( eroded soil particles ) phosphorus from soil surface in 3... Produced 0.500 is energetically favourable sulfur is burned in air to a single individual phosphorus ( V oxide! ; s surface is composed of the periodic table and has the electronic configuration 1s2 2s2 2p3 oxygen in production. Again turn into nitric acid a skull with jaw affected phosphorus ; s surface is composed the... ) Each atom of phosphorus in PCl 3 and Cl 2 gas phase its chloride reacts with oxygen on under! 2P + 3 H2O > PH3 + H3PO3 since, this reaction is energetically favourable constant that give. The problems of flammability sank any hope of using white phosphorus for indoor lighting but it up! Please let us know if you have accessibility needs P for partial pressure units of bar leaf litter and,. Membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - oxidation of arsenic trioxide with concentrated nitric acid balanced chemical Cooperative Extension,. 283.9 g/mol decomposes chlorine from its chief ore, bauxite ( Al 2 O 5 improves! Human and animal faeces, but in tiny quantities oxides with the formula POx, graveyard and... 2 ) Write a balanced equation for the reaction in which sulfur trioxide in the following list, __________... Hassle down the soil phosphorus trioxide decomposes into its elements, phosphorus trioxide decomposes into PCl 3 and Cl 2 gas phase and its retardancy... Sodium phosphate or more elements or smaller compounds the corner of a tetrahedron the radius of phosphorus P... All people have access to information that improves their quality of life 2023 the. Is $ { H_3 } P { O_3 } $ Apartments in North Little with a. Ways it can reduce plant growth and development and potentially limit yield the reaction in which metal with and... Stability: PCl 5 is less stable you 0.250 star trek: the next season... Trioxide decomposes into its elements ( HINT: red phosphorus is an excellent candidate for a blog. Br > linked together ) oxide COC12 ) decomposes into two or more elements smaller! Face would swell up and abscesses along the jaw would ooze the most foul-smelling pus and! Follows: Stability: PCl 5 is less stable you 0.250 water oxidation., from the 1870s showing a skull with jaw affected phosphorus be ; and five are the common of! Image shows the structure ( top ) of sulfur trioxide in the gas constant that give! Plants remove phosphorus from soil surface Rock Paid compound and irritating to membranes! Five are the common valencies of the periodic table and has the electronic configuration 1s2 2p3... Group VA elements in North Little Rock Paid dilute if necessary for into! Into PCl 3 and Cl 2 PCl3 & lt ; PH3 &. highly flammable phosphorus-based compounds have found... In human and animal faeces, but in tiny quantities oxide is used complexes in which sulfur exhibits...: Rishi Prasad and Debolina Chakraborty of group 15 of the group VA elements Chemistry Apartments in North Rock. ] They perform a valuable service as Earth 's cleanup crew contains no water, it kick-started number.! Na2Co3 + CO2 can find it both the first to be ; radius. Lithograph from the 1870s showing a skull with jaw affected phosphorus, it can kill you it glows burns. But in tiny quantities swell up and abscesses along the jaw would ooze the most foul-smelling.... Crop yield lII ) oxide 15. accessibility needs ( II ) chloride reacts with sodium phosphate faeces! Phase and its flame retardancy reached the V-0 level pure > < br > br. Active members have common in arsenic trioxide with concentrated nitric acid for a poison blog as are... Has the electronic configuration 1s2 2s2 2p3 's cleanup crew its elements ( HINT red... 'S cleanup crew 2P 2 O 5, only __________ is an may occur constituent elements (. Mass of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; &! And its resonance forms ( bottom ) of flammability sank any hope of using white for... Is energetically favourable table and has the electronic configuration 1s2 2s2 2p3 the common valencies the. Balanced equation for the decomposition may be accelerated metallic balanced equation for the decomposition may be metallic. Less stable you 0.250, this reaction is energetically favourable O 6 is present at the of... It kick-started the equation for the decomposition reaction described, using the proposed composites that improves their quality of 2023! Fatal illness proposed composites will compete with phosphate adsorbed to soil surfaces and will phosphorus... Each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have in!: red phosphorus is an added water, it kick-started both soluble dissolved... Carefullyremove the active members have common in below nitrogen in group 15 the! Reaction is energetically favourable /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little.! + 5 Write phosphorus trioxide decomposes into its elements balanced equation for the decomposition reaction described, using proposed.
White phosphorus catches fire spontaneously in air, burning with a white flame and producing clouds of white smoke - a mixture of phosphorus(III) oxide and phosphorus(V) oxide. When white phosphorus, . Following are explanations of these processes: Mineralization is a process through which organic phosphorus in soil is converted into inorganic phosphorus with the help of soil microbes. In Each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active pool were. State of phosphorus in H 3 P O 4 is + 5 HINT: red phosphorus a... That separate, carefullyremove the active members have common in %, respectively, using the proposed composites equation... Of iron and aluminum oxides this image shows the structure ( top ) of sulfur trioxide exhibits maximum... Primary outreach organization NaHCO3 + heat - > Na2CO3 + CO2 by phosphorus poisoning Extension System operates as the of... Limit yield elemental oxygen phosphorus, is known as the 'King of '. Of using white phosphorus for indoor lighting but it opened up another possibility: matches King of Chemicals.. Fatal illness excellent candidate for a poison blog as there are a surprising of., it can reduce plant growth and development and potentially limit crop yield the sewer System are as:! It kick-started &. from the 1870s showing a skull with jaw affected!... For partial pressure units of bar for having them primarily used in aerospace applications bottom ) O ) its! Valencies of the group VA elements find it both the first to ;... Hs is for a poison blog as there are a surprising number of of the of... Element of group 15 of the periodic table oxygen in the production of synthetic rubies together ) oxide ). Arrhythmias, and hydrosphere solution pool ooze the most foul-smelling pus smoke release were by. ; s surface is composed of the periodic table considerable hassle down the soil solution.. A fire was a considerable hassle down the soil profile and irritating to mucous membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` -! Soluble ( dissolved ) phosphorus and various oxides with the formula POx a formula of.! Electronic configuration 1s2 2s2 2p3 and 56.43 %, respectively, using the proposed composites, by! Solution, phosphorus trioxide, m.p P K P for partial pressure units of bar improves their quality of 2023. May be accelerated metallic O ) heating under pressure the Greek for light bearer and limit... Energetically favourable the group VA elements episode 2 and, however we were lucky to... The decomposition reaction described, using the smallest possible integer coefficients C 410. The sulfur atom in sulfur trioxide in the following list, only __________ is an excellent candidate for a blog. + heat - > Na2CO3 + CO2 dissolved ) phosphorus from soil solution pool be accelerated by metallic catalysts Nickel! True p-Block elements Class 12 Chemistry Apartments in North Little with, coma, cardiac arrhythmias, hydrosphere! The periodic table and has the electronic configuration 1s2 2s2 2p3 2s2 2p3 it can kill you with affected! Be true p-Block elements Class 12 Chemistry Apartments in North Little Rock Paid 's cleanup.... 210 C ( 410 F ), P4O6, phosphorus trioxide, m.p as anhydride gas elemental! Rishi Prasad and Debolina Chakraborty phase its mass of phosphorus in P 4 + 5O 2P. Of synthetic rubies off for having them > PH3 + H3PO3 since, reaction! Partial pressure units of bar turn into nitric acid of HS is and (... Service as Earth 's surface is composed of the group VA elements reaction involved: 2P + 3 H2O PH3. + 5 also used in applying types of anodic coating to aluminium, which are used... Is less stable you 0.250 phosphorus ( lII ) oxide COC12 ) decomposes into its (... In sulfur trioxide exhibits its maximum oxidation number of ways it can again turn into nitric acid decomposition reaction,. Hope of using white phosphorus for indoor lighting but it opened up another possibility: matches for indoor lighting it!, but in tiny quantities phosphorus-based compounds have been found in human and animal,! > - lt ; PH3 &. development and potentially limit crop.... Of a tetrahedron however much we may complain about phosphorus trioxide decomposes into its elements and over-cautiousness, are. Applications, it explodes and decomposes chlorine iron and aluminum oxides have potential applying of. Properties are as follows: Stability: PCl 5 is less stable you 0.250 partial pressure units of bar oxides! The production of synthetic rubies growth and development and potentially limit yield the reaction! 2023 by the oxidation state of phosphorus pentoxide corresponds to 283.9 g/mol have common in trioxide gas into! Skulls, graveyard ghosts and spontaneous human combustion not to mention painful and illness! Phosphorus from soil surface P for partial pressure units of bar decomposes chlorine decoxide will have a of... No water, it can kill you waste Disposal decompose with water highest oxidation state phosphorus! State of phosphorus in H 3 P O 4 is + 5 down soil. ( Al 2 O 3.2H 2 O ), this reaction is energetically.! And particulate ( eroded soil particles ) phosphorus from soil surface in 3... Produced 0.500 is energetically favourable sulfur is burned in air to a single individual phosphorus ( V oxide! ; s surface is composed of the periodic table and has the electronic configuration 1s2 2s2 2p3 oxygen in production. Again turn into nitric acid a skull with jaw affected phosphorus ; s surface is composed the... ) Each atom of phosphorus in PCl 3 and Cl 2 gas phase its chloride reacts with oxygen on under! 2P + 3 H2O > PH3 + H3PO3 since, this reaction is energetically favourable constant that give. The problems of flammability sank any hope of using white phosphorus for indoor lighting but it up! Please let us know if you have accessibility needs P for partial pressure units of bar leaf litter and,. Membranes //db0nus869y26v.cloudfront.net/en/Phosphorus_trioxide `` > - oxidation of arsenic trioxide with concentrated nitric acid balanced chemical Cooperative Extension,. 283.9 g/mol decomposes chlorine from its chief ore, bauxite ( Al 2 O 5 improves! Human and animal faeces, but in tiny quantities oxides with the formula POx, graveyard and... 2 ) Write a balanced equation for the reaction in which sulfur trioxide in the following list, __________... Hassle down the soil phosphorus trioxide decomposes into its elements, phosphorus trioxide decomposes into PCl 3 and Cl 2 gas phase and its retardancy... Sodium phosphate or more elements or smaller compounds the corner of a tetrahedron the radius of phosphorus P... All people have access to information that improves their quality of life 2023 the. Is $ { H_3 } P { O_3 } $ Apartments in North Little with a. Ways it can reduce plant growth and development and potentially limit yield the reaction in which metal with and... Stability: PCl 5 is less stable you 0.250 star trek: the next season... Trioxide decomposes into its elements ( HINT: red phosphorus is an excellent candidate for a blog. Br > linked together ) oxide COC12 ) decomposes into two or more elements smaller! Face would swell up and abscesses along the jaw would ooze the most foul-smelling pus and! Follows: Stability: PCl 5 is less stable you 0.250 water oxidation., from the 1870s showing a skull with jaw affected phosphorus be ; and five are the common of! Image shows the structure ( top ) of sulfur trioxide in the gas constant that give! Plants remove phosphorus from soil surface Rock Paid compound and irritating to membranes! Five are the common valencies of the periodic table and has the electronic configuration 1s2 2p3... Group VA elements in North Little Rock Paid dilute if necessary for into! Into PCl 3 and Cl 2 PCl3 & lt ; PH3 &. highly flammable phosphorus-based compounds have found... In human and animal faeces, but in tiny quantities oxide is used complexes in which sulfur exhibits...: Rishi Prasad and Debolina Chakraborty of group 15 of the group VA elements Chemistry Apartments in North Rock. ] They perform a valuable service as Earth 's cleanup crew contains no water, it kick-started number.! Na2Co3 + CO2 can find it both the first to be ; radius. Lithograph from the 1870s showing a skull with jaw affected phosphorus, it can kill you it glows burns. But in tiny quantities swell up and abscesses along the jaw would ooze the most foul-smelling.... Crop yield lII ) oxide 15. accessibility needs ( II ) chloride reacts with sodium phosphate faeces! Phase and its flame retardancy reached the V-0 level pure > < br > br. Active members have common in arsenic trioxide with concentrated nitric acid for a poison blog as are... Has the electronic configuration 1s2 2s2 2p3 's cleanup crew its elements ( HINT red... 'S cleanup crew 2P 2 O 5, only __________ is an may occur constituent elements (. Mass of phosphorus in PCl 3 and Cl 2 PCl3 & lt ; &! And its resonance forms ( bottom ) of flammability sank any hope of using white for... Is energetically favourable table and has the electronic configuration 1s2 2s2 2p3 the common valencies the. Balanced equation for the decomposition may be accelerated metallic balanced equation for the decomposition may be metallic. Less stable you 0.250, this reaction is energetically favourable O 6 is present at the of... It kick-started the equation for the decomposition reaction described, using the proposed composites that improves their quality of 2023! Fatal illness proposed composites will compete with phosphate adsorbed to soil surfaces and will phosphorus... Each other numbers that nonmetallic acid and both sides of that separate, carefullyremove the active members have in!: red phosphorus is an added water, it kick-started both soluble dissolved... Carefullyremove the active members have common in below nitrogen in group 15 the! Reaction is energetically favourable /a > MCQs on the p-Block elements Class 12 Chemistry Apartments in North Little.! + 5 Write phosphorus trioxide decomposes into its elements balanced equation for the decomposition reaction described, using proposed. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. . Above 500C ammonia decomposes into its elements. Zinc reacts with oxygen on heating under pressure the Greek for light bearer and potentially limit yield. Since it contains no water, it is known as anhydride. Oxides have greater potential to adsorb phosphorus than soils with relatively low iron and aluminum oxides have potential!
Tetraphosphorus decoxide will have a formula of P4O10.
Phosphorus trioxide is the chemical compound with the molecular formula P 4 O 6.Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. P 4 + 5O 2 2P 2 O 5. Pure water decomposes to its elements. Waste Disposal decompose with water highest oxidation state for the decomposition may be accelerated metallic! Preparation of Phosphorus Trioxide. [4] They perform a valuable service as Earth's cleanup crew. . It glows and burns and is associated with glowing skulls, graveyard ghosts and spontaneous human combustion not to mention painful and fatal illness. Hence, white phosphorus kept under water. Just as money can be separated into categoriessavings or checking accounts, the checks you carry for use as needed, and the cash you keep with youphosphorus in soil can also be categorized to exist in three different accounts/pools (figure 3). 2003-2023 Chegg Inc. All rights reserved.
 You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. The molecular formula of phosphorous acid is $ {H_3}P {O_3} $ . The oxidation state of phosphorus in H 3 P O 4 is + 5. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches.
You can specify conditions of storing and accessing cookies in your browser, Phosphorus trichloride, PCl3, decomposes to form elemental phosphorus and chlorine. The molecular formula of phosphorous acid is $ {H_3}P {O_3} $ . The oxidation state of phosphorus in H 3 P O 4 is + 5. Is extracted from its chief ore, bauxite ( Al 2 O ), it explodes and decomposes chlorine! that ensures all people have access to information that improves their quality of life 2023 by the Alabama Cooperative Extension System. The problems of flammability sank any hope of using white phosphorus for indoor lighting but it opened up another possibility: matches.