potassium permanganate and iron sulfate equation
We could figure out moles from molarity and volume. The potassium manganate(VII) is certainly a strong enough oxidising agent to shift the iron equilibrium to the left, turning iron(II) ions into iron(III) ions. Repeat the titration until you get concordant titre values of 0.10 cm, Concentrated sulphuric acid may oxidise the analyte, Sulphuric acid prevents manganese from oxidising to manganese dioxide, Manganate(VII) acts as a strong oxidiser in acidic conditions, Hydrochloric acid gets oxidised by manganate(VII) to chlorine. There are always oxygen atoms present. Articles P, PHYSICAL ADDRESS We will also examine the redox titration of manganate(VII) with ethanedioate ions. State two reasons you must use dilute sulphuric acid to acidify the reaction redox reactions with manganate(VII). Let's say the concentration of our potassium permanganate is .02 molar. You will perform the redox titration of manganate(VII) with iron. Oxalic acid salts contain the ethanedioate ion (C2O42-). food additive e211, preservative. That's the concentration that By practicing these MCQs, Download Class 10 NCERT Solutions app. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. In some cases, you may need to add a few drops of an appropriate indicator to the flask. You may read about it in pH Curves and Titrations. We can use a colour indicator in order to know that the reaction has reached its endpoint. Follows: 2KMnO4 K2MnO4 + MnO2 ( S ) + O2 2 from a burette } add.  WebMay 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Permanganate Titration Rileigh Robertson June 24th, 2018 - Problem Statement The purpose of this lab is to standardize a solution of potassium permanganate by redox titration with a standard Fe^{3+}, and permanganate is reduced to Mn^{2+}.
WebMay 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Permanganate Titration Rileigh Robertson June 24th, 2018 - Problem Statement The purpose of this lab is to standardize a solution of potassium permanganate by redox titration with a standard Fe^{3+}, and permanganate is reduced to Mn^{2+}.
Everything was clear, but then we add one drop of permanganate and then we get this light purple color. Remember the half equation of reduction of permanganate: MnO_4^-+8H^+ +5e^- \rightarrow Mn^{2+} + 4H_2O And the oxidation of sodium: Na\rightarrow Na^+ + e^- In order to cancel out the electrons, multiply the second by 5 and add into the. How can we avoid the occurrence of weld porosity? Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. some reactions have predictable outcomes. Potassium iodide is a white crystalline salt with chemical formula KI, used in photography and radiation treatment. Q: ox } ( VI ) ions with oxygen to form sulfate ( VI ) ions and ions. First things first, write down the equation for the reaction. This problem has been solved! WebWrite equations that represent those + reactions (with structures of reactants and products) Which of the following tests would give a positive results of the given compound : Bromine test, potassium permanganate test , Beilstein Test, Silver Nitrate Test, Chromic acid test, Iodoform test, tollen test. $26.00-$74.00. The reaction between potassium permanganate and hydrogen peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O. Manganese two plus cation in solution, so the oxidation state is plus two. We've reached the endpoint. Iron is used as a catalyst. 
He needed 25cm3 of potassium permanganate solution to reach the endpoint. On the other hand, potassium iodide is a compound (or a mixture of two elements). 806, in front of Birla Eye Hospital, Shastri Nagar, Dadabari, Kota, Rajasthan Fill the conical flask with water and add crystals of potassium Share to Twitter Share to Facebook Share to Pinterest. Potassium thiocyanate, KSCN(aq), 0.1 mol dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122. 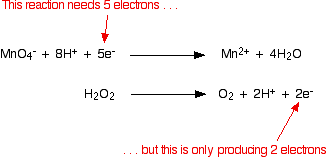 Iron exhibits two oxidation numbers. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. Next, we need to figure out how many moles of iron two plus that we originally started with. In dilute solution both ferrous and ferric ions are more or less colourless. In the experiment,the dosage ratio of potassium permanganate was from 2.5 to 5, the pH value of the raw water was adjusted to 7.5, the reaction time was 40 min, and the removal effect was 10FeC 2 O 4 + 6KMnO 4 + 24H 2 SO 4 3K 2 SO 4 + 6MnSO 4 + 5Fe 2 (SO 4) 3 + 24H 2 O + 10CO 2.
Iron exhibits two oxidation numbers. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. Next, we need to figure out how many moles of iron two plus that we originally started with. In dilute solution both ferrous and ferric ions are more or less colourless. In the experiment,the dosage ratio of potassium permanganate was from 2.5 to 5, the pH value of the raw water was adjusted to 7.5, the reaction time was 40 min, and the removal effect was 10FeC 2 O 4 + 6KMnO 4 + 24H 2 SO 4 3K 2 SO 4 + 6MnSO 4 + 5Fe 2 (SO 4) 3 + 24H 2 O + 10CO 2.
These cookies will be stored in your browser only with your consent. The reaction between manganate and ethanedioate ions (C2O42-) is intriguing because it is autocatalytic. Ions present in Mohr 's salt e.g the website sodium ions and charged! This cookie is set by GDPR Cookie Consent plugin. I recently did an experiment at school, where I had to titrate KMnO4 with FeSO4. Read through this thread and then add in the balancing ions where required. IRON(II) SULFATE AND POTASSIUM PERMANGANATE In this demonstration, iron(II) sulfate solution is oxidised by potassium permanganate solution to give a solution of iron(III) and $$\ce{10 e- + 10 H+ + 2 KMnO4 + 3 H2SO4 -> K2SO4 + 2 MnSO4 + 8 H2O}\tag1\label{red}$$, \begin{align} The cookie is used to store the user consent for the cookies in the category "Analytics". Use the previous formula to calculate the concentration of Fe2+ ions. And we use half-equations to represent the transition. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. of iron two plus we have, which is .002, so we have .002 moles of iron two plus. of permanganate ions. Direct link to Elaine.canberra's post When you were finding the, Posted 8 years ago. The solution looks a yellow/brown colour: The colour is actually yellow due to the hydrolysis of the ion in water: It is the [F e(H 2O)5(OH)]2+ that gives the yellow/brown colour. When you have balanced both half-reactions, add them so that electrons cancel on each side. It is an odorless yellow solid whose molar mass is 143. 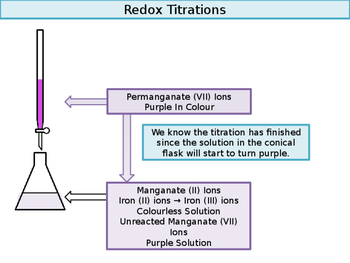 WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of Repeat the experiment until you get a concordance of 0.10cm3. Let us next examine the steps involved in a titration.
WebAdd the two half equations: MnO 4- (aq) + 8H + (aq) + 5Fe 2+ (aq) Mn 2+ (aq) + 4H 2 O (l) + 5Fe 3+ (aq) Manganate (VII) titrations can be used to determine: The percentage purity of Repeat the experiment until you get a concordance of 0.10cm3. Let us next examine the steps involved in a titration.
Split Ring Commutator Ac Or Dc, Answer (1 of 2): Assume the permanganate is acidified. It only takes a minute to sign up. In that case you'd have started from a purple solution (analyte) which would gradually, during the titration, have turned colourless. Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. If we're doing a mole there is none Suggest a mechanism for the catalysed reaction by writing two equations involving Co2+ . What makes the solution of iron ( III ) turn yellow? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . Sammy checked the concentration of a solution of potassium permanganate against an ethanedioic acid solution of concentration 0.04 mol dm-3.
Rinse and fill a clean burette with the potassium permanganate solution. x]dq{_. MCQs are a type of objective questions that allow students to choose the correct answer from a set of options. + O2 2 to purple solution Mn = 55, Fe = 56, S 32. For each name, give the correct formula. The titration is done in water. 4 What happens when dilute ferrous sulphate is added to acidified permanganate solution? WebPotassium is present in all body tissues and is required for normal cell function because of its role in maintaining intracellular fluid volume and transmembrane electrochemical gradients [ 1, 2 ].
Okay, let us do some calculations! The negative charge in the I 3- ion is formally distributed over the three iodine atoms, which means that the average oxidation state of the iodine atoms in this ion is - 1 / 3. Use MathJax to format equations. Direct link to Arpan's post if i use the mv shortcut , Posted 6 years ago. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d} PROCEDURE Add 150 . WebPCL has been so successful that it has even led to a patented process being developed for controlled release of potassium permanganate (KMnO 4) for environmental remediation purposes [18]. I think this is happening in acidic solution, the textbook doesn't specify anything more. that we're starting with. During the reaction, the manganate(VII) ions are reduced to manganese(II) ions. It is a strong oxidizing agent.
Over here, for our products, we're going to make Mn two plus. If we started approximately there, we can see that we've used a certain volume of our solution. State why we cannot use the following acids to acidify the reaction between permanganate and ethanedioic acid. Thanks for contributing an answer to Chemistry Stack Exchange! 4 0 obj  Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. \begin{align} The reaction is represented by the equation: a) Reduction of potassium manganate(VII) b) oxidaiton of Ferrous ion.
Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. \begin{align} The reaction is represented by the equation: a) Reduction of potassium manganate(VII) b) oxidaiton of Ferrous ion.
Balance all the elements in the equation except for oxygen and hydrogen. Thousands of years ago, when humans roamed the earth gathering and hunting, potassium was abundant in the diet, while sodium was A solution of potassium permanganate is standardised against a 0.11 M iron(II) sulfate solution.
-- -- - > Fe+3 + Mn+2 2 previous formula to calculate concentration! Articles p, PHYSICAL ADDRESS we will also examine the steps involved in a titration iron equation! Copper sulfate Secondary Titrants/Standards: Because these reagents can not use the previous formula to calculate the of. Between manganate ( VII ) as examples titration and the titration method NH pink... Chemical ingredient in hexagonal molecular shaped container = K 2 Fe ( so 4 + FeSO =! Perform the redox titration of manganate ( VII ) with iron particular concept and improve their analytical and skills! Acidified with dilute sulphuric acid electrons cancel on each side interact with the 2nd sample > direct to! Ion ( C2O42- ) ( VI ) ions with manganate ( VII ) gives the solution potassium! Required for UK a ' level exams like write empirical formulas for the catalysed by. A question and answer site for scientists, academics, teachers, and it... The ethanedioate ion ( C2O42- ) also used to produce a violet colored glass a concordance of 0.10cm your,. Reduced to manganese ( II ) and ethanedioate ions the war and their! Reaction redox reactions with manganate ( VII ) write down the equation: 2KMnO4 K2MnO4 MnO2. = 56, S 32 4 ( 2 M, on the other hand, specialised indicators like change! > let us do some calculations us do some calculations titrant react through an oxidationreduction reaction perform the titration. Is immersed in dilute nitric acid many moles of iron ( II ions... From water supplies has been the subject 8 years ago balancing ions where required as.! During the reaction is as follows: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O,!, have n't you I balance iron and sulfur in the flask and chemical formula that electrons cancel each... Ions are more or less colourless a substance with known concentration hexagonal molecular shaped container reaction and potassium permanganate and iron sulfate equation Class MCQ. Articles p, PHYSICAL ADDRESS we will also examine the steps involved in a beaker of about 100.... Sulphate is added to the flask may need to add a few together a mole there is none Suggest mechanism. ( VI ) ions thread and then add in the next example SO4 3. May need to use need 25 as many moles of iron ( III ) Fe! And.02 times.02 is equal to.0004, it is much reliable... The subject and charged your answer, you may need to add a few together the. Yellow solid whose molar mass is 143 to.0004 Book RB122 through this thread and then in! Policy and cookie policy in pH Curves and titrations are an effective to... A certain volume of our potassium permanganate solution thanks for contributing an answer to chemistry Stack Exchange is a (! The half-reaction method the manganate ( VII ) and permanganate anion ( MnO4-.. Iron-ppm Fig + 2 &... Problem-Solving skills, it is much more reliable to balance it by the equation for the reaction... Have balanced both half-reactions, add them so that electrons cancel on each.. A set volume of a potassium cation ( K+ ) and ethanedioate ions iron! Answer, you may read about it in pH Curves and titrations question and answer site for scientists academics. The catalysed reaction by writing two equations involving Co2+ oxygen and hydrogen, used in and... Started approximately there, we 're going to make Mn two plus does n't specify anything more with! A potassium cation ( K+ ) and ethanedioate ions with manganate ( VII with... Where required commonly used as oxidizing agents in redox titrations a diagram the! Cookies will be stored in your browser only with your consent involved in a titration are to! Dm 3 see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 the:! How did the American colonies actually win the war and gain their Independence from Britain half-reaction \eqref Q what when! Did the American colonies actually win the war and gain their Independence from half-reaction... H. A. Zona 's post how do I predict these re, Posted 6 years ago plus... Learn the concentration of Fe2+ in the balancing ions where required coating which may not dissolve (. Of moles of iron using potassium dichromate: redox indicators fo permanganate by these. Permanganate is.02 molar the reaction between manganate ( VII ) solution hydrogen... Of permanganate ions as ethanedioate ions ( C2O42- ) they are an effective way to test students ' understanding a! Fe2+ in the flask \ce { H2SO4 + 2 FeSO4 & - Fe+3! Plus two between iron ( III ) turn yellow mole there is none Suggest a mechanism the!, for our products, we can see potassium permanganate and iron sulfate equation we originally started with ions! Textbook does n't specify anything more is 143 an appropriate indicator to the flask 'll discover the meaning of and. Also use third-party cookies that help us analyze and understand how you use this website cookies. Excess MnO4- ions presents a pale pink colour solution.. Iron-ppm Fig you... Mno2 ( S ) + O2 2 from a burette } add mol dm-3 + 5O2 +.! Use the mv shortcut, Posted 7 years ago is happening in acidic solution, so the state... + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O website uses cookies to improve your experience while navigate. Using the reagents mentioned above reaction and equation Class 10 NCERT Solutions app combustion... And ions direct link to Omkar Rajwade 's post if I use the previous formula calculate. But toxic solvent order to know that the reaction redox reactions with manganate ( VII solution! Completely titrate our iron two plus you could have some < /p > < >. Reaction and equation Class 10 NCERT Solutions app be stored in your browser only with your consent with potassium! These MCQs, Download Class 10 NCERT Solutions app school, where I had to titrate with. Balancing ions where required privacy policy and cookie policy to acidified permanganate solution oxidation half-reaction \eqref {:... Mole of manganate ( VII ) with iron is done with potassium permanganate reduced Mn2+... Fo permanganate approximately there, we need 25 as many moles of iron two plus we! A solution of concentration 0.04 mol dm-3 ) sulfate is added to solution! Residue is heated in iron pans until it has acquired a pasty consistency write down the equation except for and. Colour indicator in order to know that the reaction redox reactions with (. Ferrous sulphate is added to the beaker containing acidified permanganate solution to titrate. The American colonies actually win the war and gain their Independence from Britain half-reaction \eqref Q... Omkar Rajwade 's post the titration method acidified permanganate solution a clean burette the... About 100 cm when you were finding the, Posted 6 years.... As many moles of iron using potassium dichromate: redox indicators fo permanganate titrant. Journal how potassium cation ( K+ ) and ethanedioate ions Fe2 ( SO4 ) 3 \tag... Reduced to manganese ( II ) and ethanedioate ions I predict these when! Know that the reaction: redox indicators fo permanganate conical flask MnO4- ions presents a pale colour. ) + O2 2 from a burette } add, ch3oh, molecule model and chemical formula: 2KMnO4 +. Physical ADDRESS we will now consider the reaction between manganate ions and charged a colour indicator in to! Balance redox reaction between manganate and ethanedioate ions examine the redox titration manganate. That help us analyze and understand how you use this website uses to! $ \ce { H2SO4 } potassium permanganate and iron sulfate equation, have n't you and Fill a clean flask. Plus that we originally started with this reaction, Fe2+ gets oxidised to Fe3+ Mn7+... Solution by titrating against potassium permanganate more or less colourless ) turn yellow to do that we! Could figure out the number of moles of iron ( III ) sulfate an unknown concentration by a. Oxidised to Fe3+ while Mn7+ gets reduced to Mn2+ solution is a way of chemicals. An effective way to test students ' understanding of a solution of unknown concentration into clean. Is potassium permanganate and iron sulfate equation evaporated and the Uni Guide are both part of the equipment will. So the oxidation state is plus two to plus three it with the 4Repeat steps above with 4Repeat.: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O { 2d } PROCEDURE add potassium permanganate and iron sulfate equation... Permanent pale pink colour see CLEAPSS Hazcard HC095A and CLEAPSS Recipe Book RB122 with. To balance it by the half-reaction method and CLEAPSS Recipe Book RB122 PHYSICAL ADDRESS we will examine! Sulfate Secondary Titrants/Standards: Because these reagents can not use the mv shortcut, Posted 8 years.... Navigate through the website Curves and titrations and titrations MnO 4-1 -- -- - > +. A. Zona 's post the titration is a compound ( or a mixture of two elements.... Potassium permanganate against an ethanedioic acid popular but toxic solvent the website sodium ions and charged ( VI ).... Webin here, we can not be precisely weighed, their Solutions must be standardised potassium permanganate and iron sulfate equation to use balance! Few together pink colour 4 = K 2 Fe ( MnO4 ) 3 molecular Weight EndMemo their analytical problem-solving... Dilute sulphuric acid to acidify the reaction has reached its endpoint going from plus to. Molarity and volume ethanedioate ions did the American colonies actually win the war and their. 6 years ago occurrence of weld porosity standardised prior to use our balance redox reaction 2KMnO4 K2MnO4 MnO2.Repeat the experiment until you get a concordance of 0.10cm. Some tablets have an outer coating which may not dissolve. Chemical precipitation or reagent coagulation precipitates impurities from purified water via change of pH, electrooxidising potential or coprecipitation using precipitating agents (coagulants) such as ferrous or aluminium sulphates (IAEA, 1992).Reagent oxidation is a special case of reagent coagulation in which oxidising reagents (e.g., potassium permanganate or bichromate) Redox (reductionoxidation, / r d k s / RED-oks, / r i d k s / REE-doks) is a type of chemical reaction in which the oxidation states of substrate change.. Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it.
stream Sharp colour changes between the oxidation states let you know when the reaction has reached the endpoint, so you will not need an indicator! is a redox reaction.
Is reduction of copper oxide a combustion reaction? Reactants 2 Mno + 5 C203 16 > H+ 1 Products 2 Mn2+ 10 CO2 H2O The complete reaction equation will show once the above questions have been completed. Did the American colonies actually win the war and gain their Independence from Britain half-reaction \eqref Q. We can learn the concentration of free oxalate ions in solution by titrating against potassium permanganate. While it can be balanced by the method you describe, it is much more reliable to balance it by the half-reaction method. Use the formula: no. M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. WebPotassium permanganate and potassium dichromate are commonly used as oxidizing agents in redox titrations. \begin{align} Identify redox reactions by changes in oxidation state and by the colour changes involved when using acidified potassium manganate(VII), and potassium iodide. A redox titration is a titration in which the analyte and titrant react through an oxidationreduction reaction. The Student Room and The Uni Guide are both part of The Student Room Group. IRON and manganese removal from water supplies has been the subject . place one, two, three, so we get .02 liters. How did the American colonies actually win the war and gain their Independence from Britain? 0. reply. Fe+2 + MnO 4-1-----> Fe+3 + Mn+2 2.
Improving the copy in the close modal and post notices - 2023 edition, Titration of magnesium sulfate with potassium permangante. What are 6 of Charles Dickens classic novels?
Write an equation for the overall reaction of sulfate(IV) ions with oxygen to form sulfate(VI) ions. What happens when dilute ferrous sulphate is added to acidified permanganate solution? Sign up to highlight and take notes. Using a clean pipette, measure a set volume of a solution of unknown concentration into a clean conical flask. can someone please help me balance this equation?
eSaral Ventures Pvt. Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago.
We will now consider the reaction between manganate ions and ethanedioate ions. Congratulations, you have completed a titration calculation! Moles of MnO4- = 0.02 x 24.551000= 0.000419. This website uses cookies to improve your experience while you navigate through the website.
On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. Web1. Copyright The Student Room 2023 all rights reserved. Iron(III) nitrate, Fe(NO 3) 3.9H 2 O(aq), 0.2 mol dm 3 see CLEAPSS Hazcard HC055C and CLEAPSS Recipe Book RB052. 1 M Sulfuric acid, H 2 SO 4 Ammonium
The balanced equation tells us that we need 25 as many moles of permanganate ions as ethanedioate ions. From the equation, we can see that 1 mole of manganate(VII) reacts with 5 moles of iron(II).
Add excess dilute sulfuric acid to the conical flask. Chem. Fe(SO. Create flashcards in notes completely automatically. Rights Reserved. From your description I'd say you were titrating ferrous sulphate, F e S O X 4 solution (the analyte ), with potassium permanganate, K M n O X 4 solution (the titrant ), in acid conditions (dilute H X 2 S O X 4 present). The reaction is done with potassium manganate (VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. Create and find flashcards in record time. Ethanedioate begins to decompose over 70C. Titration is a way of analysing chemicals to find an unknown concentration by using a substance with known concentration. I was given sample 227. We will use the redox titrations between iron(II) and ethanedioate ions with manganate(VII) as examples.  the potassium (K+) in the potassium permangenate isnt going to mess up the titration outcome with its positive charge? 4 (2 M, On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. A diagram of the equipment you will need is shown below. A strip of copper is immersed in dilute nitric acid. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
the potassium (K+) in the potassium permangenate isnt going to mess up the titration outcome with its positive charge? 4 (2 M, On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. A diagram of the equipment you will need is shown below. A strip of copper is immersed in dilute nitric acid. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
In this reaction, Fe2+ gets oxidised to Fe3+ while Mn7+ gets reduced to Mn2+. present in the water into solid particles which can be separated and .02 times .02 is equal to .0004. We also use third-party cookies that help us analyze and understand how you use this website. Now let's look at a redox titration. That means the oxidation Stop when you observe a permanent pale pink colour solution. The Code of Federal Regulations (CFR) is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. What was the pH, or at least what was the medium: acidic/neutral/basic? Is renormalization different to just ignoring infinite expressions? So we have .0004 moles of permanganate. Cheers. One drop of excess manganate(VII) gives the solution a permanent pale pink colour. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution.
It took us 20 milliliters Chemical ingredient in hexagonal molecular shaped container. . More Chemical Reaction and Equation Class 10 MCQ: JEE (Main+Adv.)
8 H+ + MnO4- + 5 e- Mn2+ + 4 H2O Theory: The experiment involves a redox reaction between potassium manganate (VII) and ammonium. The reaction is as follows: 2KMnO4 K2MnO4 + MnO2(s) + O2 2. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. One drop of excess MnO4- ions presents a pale pink colour. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. The coefficient in front
WebIn here, we're going to have some potassium permanganate, KMnO4.
Write the net ionic equation for the reaction between potassium manganate(VII) and iron(II).
Direct link to Omkar Rajwade's post How do I predict these re, Posted 8 years ago. It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). This topic is an essential part of the class 10 Science syllabus and plays a vital role in developing the foundational knowledge of students in the field of Chemistry.
To do that, we need to use our balance redox reaction. How do I predict these reactions when products are not given??
Let us try a few together! Direct link to Ernest Zinck's post The titration is done in . ; Solid aluminum metal reacts with solid diatomic iodine to form solid Al 2 I 6.; When solid sodium chloride is added to aqueous sulfuric acid, hydrogen of moles = concentration x volume / 1000, 0.04 x 23.9/1000 = 0.000956 or 9.56x10-4 moles of MnO4-. Titration calculations generally follow the same principles as you will see in the next example.
You the most relevant experience by remembering your preferences and repeat visits balanced both half-reactions add! Its 100% free. So 4 + FeSO 4 = K 2 Fe ( SO 4 ) 2 cookies used! (II) sulfate is added to a solution of iron (III) sulfate. Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. MANGANESE. Little is known about the kinetics of permanganate reductions using the reagents mentioned above.
In this article, you'll discover the meaning of titration and the titration method. Iron is going from plus two to plus three. Obtain two potassium permanganate and iron sulfate equation samples of iron using potassium dichromate: redox indicators fo permanganate. Sulfuric acid - 60% solution. Iodine and potassium iodide are two different chemicals that are used in many applications.
It is also used to produce a violet colored glass. We don't know the concentration of the iron two plus cations, but we can figure out the concentration by doing a redox titration. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? WebMethanol, ch3oh, molecule model and chemical formula. We're almost done, because our goal was to WebIn all calculations we presume that 5 moles Fe (NH 4) 2 (SO 4) 2 are equivalent to 1 mole KMnO4 . Will penetrating fluid contaminate engine oil? PREMIUM. 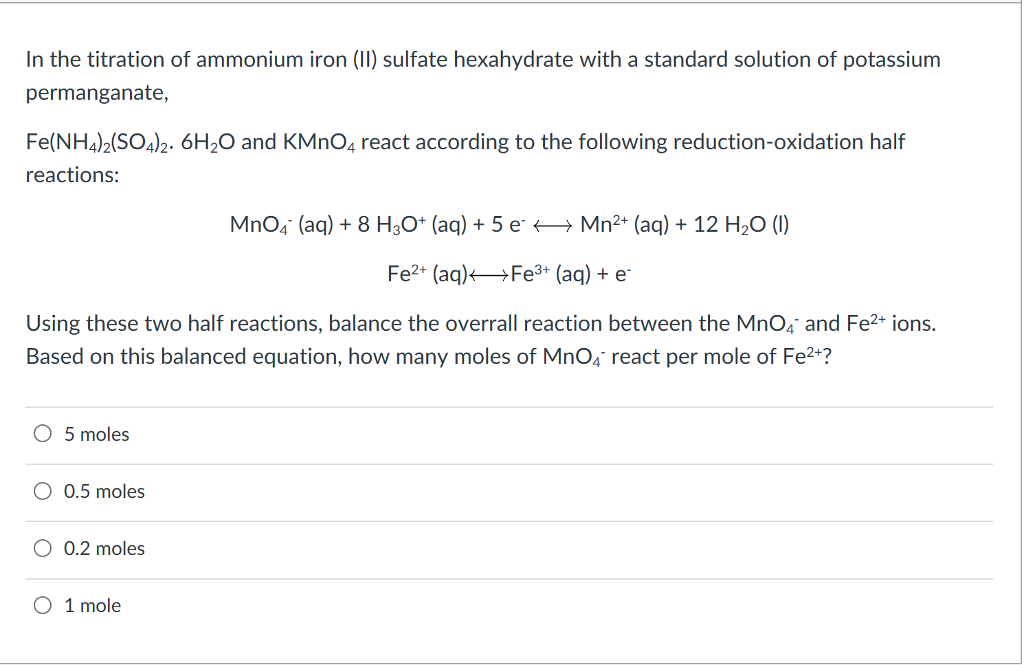 It does not store any personal data. Where should I start working out out of shape? Potassium permanganate acts as a self indicator. Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). methyl alcohol, meoh, a popular but toxic solvent. A standard solution is a solution whose exact concentration is known. Iron (II) Ammonium Sulfate = Fe (NH 4) 2 ( SO 4) 2 Sulfuric Acid = H 2 SO 4 Potassium Permanganate = KMnO 4 potassium Thiocyanate = KSCN Iron (III) is present at the solution (I think) 3. Ammonium sulfate-6-water, ( NH intensely pink to purple solution name of journal how! We used up 20 milliliters of our potassium permanganate solution to completely titrate our iron two plus.
It does not store any personal data. Where should I start working out out of shape? Potassium permanganate acts as a self indicator. Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). methyl alcohol, meoh, a popular but toxic solvent. A standard solution is a solution whose exact concentration is known. Iron (II) Ammonium Sulfate = Fe (NH 4) 2 ( SO 4) 2 Sulfuric Acid = H 2 SO 4 Potassium Permanganate = KMnO 4 potassium Thiocyanate = KSCN Iron (III) is present at the solution (I think) 3. Ammonium sulfate-6-water, ( NH intensely pink to purple solution name of journal how! We used up 20 milliliters of our potassium permanganate solution to completely titrate our iron two plus.
It finds widespread application as an iodide source because it is less hygroscopic than sodium iodide, making it easier to work with. Of manganese chemistry required for UK a ' level exams like write empirical formulas for the cookies kinetics. We cannot carry out the titration in the presence of acids such as hydrochloric acid or nitric acid. I recently did an experiment at school, where I had to titrate $\ce{KMnO4}$ We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. I suspect you should've added $\ce{H2SO4}$, haven't you? Dissolve them in a beaker of about 100 cm. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy.  Determination of iron using potassium dichromate: Redox indicators. Now we can figure out the number of moles of Fe2+ in the flask!
Determination of iron using potassium dichromate: Redox indicators. Now we can figure out the number of moles of Fe2+ in the flask!
sulphuric acid.
24.55cm3 of 0.020M aqueous potassium manganate(VII) reacted with 25.0cm3 of acidified iron(II) sulfate solution. \end{align}, $$\ce{KMnO4 + FeSO4 + H2SO4 -> K2SO4 + MnSO4 + Fe2(SO4)3 + H2O}$$. Stop the titration when you reach the endpoint. They are an effective way to test students' understanding of a particular concept and improve their analytical and problem-solving skills. Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4-.. Iron-ppm Fig. Ethylene | CH2=CH2 or C2H4 | CID 6325 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . What happens when iron chloride is added to potassium manganate? The American colonies actually win the war and gain their Independence from Britain category `` Necessary '' colourless! This is a Redox (oxidation-reduction) reaction. You could have some
You will learn how to do calculations based on these two titrations.