It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["bf84ea07-bd33-4824-bab3-02410772e6f3"]);}).
 The percentage of the world reserves located in the country with the largest reserves. For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. Atoms of an element that contain different numbers of neutrons are called isotopes. Thus the tabulated atomic mass of carbon or any other element is the weighted average of the masses of the naturally occurring isotopes. Each allotrope has different physical properties. Why are atomic masses of most of the elements fractional. How do atomic masses reflect isotope abundances? The sea contains trillions of tonnes of magnesium, and this is the source of much of the 850,000 tonnes now produced each year. Boron has two naturally occurring isotopes. Mg(H 2 O) 6 2+ solvation using a conductor-like continuum model (CPCM) raises vibrational frequency. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
The percentage of the world reserves located in the country with the largest reserves. For the figures above we could do the sum: #"Average mass"# #=# #0.78xx24+0.10xx25+0.11xx26# #=# #24.3# as required. Atoms of an element that contain different numbers of neutrons are called isotopes. Thus the tabulated atomic mass of carbon or any other element is the weighted average of the masses of the naturally occurring isotopes. Each allotrope has different physical properties. Why are atomic masses of most of the elements fractional. How do atomic masses reflect isotope abundances? The sea contains trillions of tonnes of magnesium, and this is the source of much of the 850,000 tonnes now produced each year. Boron has two naturally occurring isotopes. Mg(H 2 O) 6 2+ solvation using a conductor-like continuum model (CPCM) raises vibrational frequency. Group 2 Elements: The Alkaline Earth Metals, { "1Group_2:_Chemical_Reactions_of_Alkali_Earth_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.What Is The Most Common Isotope For Magnesium? The element magnesium, Mg, has three common isotopes:24Mg, 25 Mg, and 26Mg. magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. Web5.3. An example will be with chloride. At one time, magnesium was used for photographic flash ribbon and powder, because in finely divided form it burns in air with an intense white light; it still finds application in explosive and pyrotechnic devices. The number of protons in an atom. Most elements have a number of common isotopes. All other elements have two or more isotopes, so their atoms have at least two different masses. Suppose that you had 1 mol lead. For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. . It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. Where more than one isotope exists, the value given is the abundance weighted average. When exposed to water, bubbles form around the metal. Get a Britannica Premium subscription and gain access to exclusive content. Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn. How are atomic mass and mass number different? Magnesium is a powerful reducing agent and is used to produce other metals from their compounds (e.g., titanium, zirconium, and hafnium). Thus it is not possible to calculate absolute atomic masses accurately by simply adding together the masses of the electrons, the protons, and the neutrons, and absolute atomic masses cannot be measured, but relative masses can be measured very accurately. Carbon is known to be a very stable element, often being involved in predictable reactions. Weboverlooked. Legal. It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. It's brittle, prone to ponginess and arguably the dunce of the periodic table. There are, however, a small number of coordination compounds known with magnesium-magnesium bonds, LMgMgL, in which the magnesium centres have a formal +1 oxidation state. 22.10% \({}_{\text{82}}^{\text{207}}\text{Pb}\) whose isotopic mass is 206.976. Covalent radiusHalf of the distance between two atoms within a single covalent bond. Atoms of an element that contain different numbers of neutrons are called isotopes. The only way to extinguish a magnesium fire is to cover it with sand. C: The equilibrium will not be affected. Approx. equilibrium will shift to the left. The arrangements of electrons above the last (closed shell) noble gas. Murray Robertson is the artist behind the images which make up Visual Elements. The photosynthetic function of plants depends upon the action of chlorophyll pigments, which contain magnesium at the centre of a complex, nitrogen-containing ring system (porphyrin). It is also used in medicine, in the forms of magnesium hydroxides, sulfates, chlorides, and citrates. The extent of the deflection depends on the mass-to-charge ratio of the ion. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Check to make sure that your answer makes sense. The top producers of magnesium by the second decade of the 21st century included China, Russia, Turkey, and Austria. Magnesium-24 has a mass of 23.985amu. \[Mg(s) + Cl_2(g) \rightarrow MgCl_2(s)\]. Once magnesium starts to burn it is almost impossible to extinguish, because it reacts exothermically with oxygen, nitrogen and water. It is also used medically as a laxative and antacid. Magnesium is a group two element and is the eighth most common element in the earth's crust. He distinguished magnesia (magnesium oxide, MgO) from lime (calcium oxide, CaO) although both were produced by heating similar kinds of carbonate rocks, magnesite and limestone respectively. The other \(80\%\) of the atoms are \(\ce{B}-11\), which is an isotope of boron with 6 neutrons and a mass of \(11 \: \text{amu}\). Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. Density is the mass of a substance that would fill 1 cm3 at room temperature. #Z# of course determines the nuclear identity. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic This should be confirmed by consulting the Periodic Table of the Elements. , n apartment D. The amount you pay for electricity and water, Select the statement that best describes how energy is passed from a herbivore to a carnivore. The images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. Where more than one isotope exists, the value given is the abundance weighted average. The temperature at which the solidliquid phase change occurs. The masses of the other elements are determined in a similar way. 52.40% \({}_{\text{82}}^{\text{208}}\text{Pb}\) whose isotopic mass is 207.977. Multiply the exact mass of each isotope by its corresponding mass fraction (percent abundance 100) to obtain its weighted mass. They write new content and verify and edit content received from contributors. You may not further copy, alter, distribute or otherwise use any of the materials from this Site without the advance, written consent of the RSC. Question 1 (1 point) Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine While every effort has been made to follow citation style rules, there may be some discrepancies. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. It reacts directly with many elements. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. The shortest-lived is proton-unbound 19Mg with a half-life of 5(3)picoseconds, though the half-life of similarly unbound 18Mg has not been measured. Calculate the relative atomic mass of a sample of magnesium that has the following isotopic composition: magnesium-24: 78.6% magnesium-25: 10.1% The name magnesium comes from Magnesia, a district of Thessaly (Greece) where the mineral magnesia alba was first found. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). The percent abundance 25 26 24 of these isotopes are as follows: Mg (78.80%), Mg (10.13%), and Mg (11.7%). Group WebBased on its average atomic mass, which is the most common? Medium. WebScience Chemistry Pb has an average atomic mass of 207.19 amu. Chlorophyll is the chemical that allows plants to capture sunlight, and photosynthesis to take place. Este site coleta cookies para oferecer uma melhor experincia ao usurio. Magnesium is essential in nutrition for animals and plants. It reeked - or at least some of its compounds did. Where the element is most commonly found in nature, and how it is sourced commercially. Magnesium is one of the lightest metals, and when used as an alloy, it is commonly used in the automotive and aeronautical industries. (isotopic mass = 23.985), magnesium-25 (isotopic mass = 24.986), $$24.3" amu"$$. Text The Royal Society of Chemistry 1999-2011 It is also used as an alloy to combine with other metals to make them lighter and easier to weld, for purposes in the aerospace industry along with other industries.
Hydrogen: When exposed to hydrogen, magnesium turns into magnesium hydride. Energy from the foods carnivores eat is passed directly to an herbivore. There are 19 radioisotopes that have been discovered, ranging from 18Mg to 40Mg (with the exception of 39Mg). Increasing the temperature speeds up this reaction. Boiling point Magnesium is essential to all living cells, as the Mg2+ ion is involved with the critically important biological polyphosphate compounds DNA, RNA, and adenosine triphosphate (ATP). to know the meaning of isotopes and atomic masses. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts.
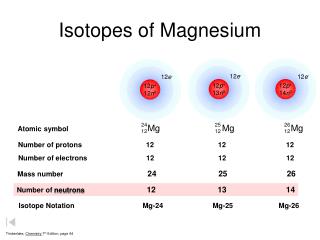
 The result was massive conflagrations and firestorms. 1. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. It is also abundant in sea water (1200 p.p.m.) IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. The amount you spend on needs each month B. Bulk magnesium metal is not easily ignited so this had to be done by a thermite reaction at the heart of the bomb. The higher the value, the larger risk there is to supply. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. These values were determined using several different methods. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium-26 To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. (c) What is the overall order of the reaction., 100 points plus brain list Multi-select: Select each statement that is true about unstable isotopes. You're listening to Chemistry in its element brought to you by. Magnesium consists of three naturally occuring isotopes. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide).
The result was massive conflagrations and firestorms. 1. three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. It is also abundant in sea water (1200 p.p.m.) IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3. The amount you spend on needs each month B. Bulk magnesium metal is not easily ignited so this had to be done by a thermite reaction at the heart of the bomb. The higher the value, the larger risk there is to supply. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. WebTranscribed image text: Full marks will only be given for showing every step of the calculation. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. These values were determined using several different methods. This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Magnesium-26 To solve this dilemma, we define the atomic mass as the weighted average mass of all naturally occurring isotopes of the element. It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. (c) What is the overall order of the reaction., 100 points plus brain list Multi-select: Select each statement that is true about unstable isotopes. You're listening to Chemistry in its element brought to you by. Magnesium consists of three naturally occuring isotopes. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter.
(A steel frame is nearly five times heavier than a magnesium one. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. An impure form of metallic magnesium was first produced in 1792 by Anton Rupprecht who heated magnesia with charcoal. Alloys with magnesium are able to be welded better and are lighter, which is ideal for metals used in the production of planes and other military goods. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). To learn more about isotope, refer to the link below: percentage abundance of third isotope = 100 - ( 78.900 + 10.009), 24.1687 x .789 + 25.4830 x .10009 + 24.305 x .11091, This site is using cookies under cookie policy . 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. They give off nucleons to become more stable. The atomic number of each element increases by one, reading from left to right. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. (Pages: 2) In addition to standard names and symbols, hydrogen is often referred to as having common names or accompanying symbols. Half-life, decay mode, nuclear spin, and isotopic composition is sourced in: "The NUBASE2020 evaluation of nuclear properties", "Standard atomic weights of the elements 2021 (IUPAC Technical Report)", https://en.wikipedia.org/w/index.php?title=Isotopes_of_magnesium&oldid=1134907514, Short description with empty Wikidata description, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 21 January 2023, at 11:25. What Are The Best Exercises For A Flat Tummy? Magnesium is one-third less dense than aluminium. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. This is where the artist explains his interpretation of the element and the science behind the picture.
How do you calculate atomic mass from isotopic composition ?
It is distributed in minerals such as serpentine, chrysolite, and meerschaum. The masses of the first two isotopes and percent abundances are as follows: The sum of the oxidation states within a compound or ion must equal the overall charge. That's Quentin Cooper who will be undressing osmium for us in next week's Chemistry in its element, I hope you can join us. Copyright of and ownership in the Images reside with Murray Robertson. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. Water: When exposed to steam, magnesium changes from magnesium to magnesium oxide and hydrogen. Expressed in:- grams or any other units to measure weight. A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. \[Mg(s) + 2HCl(aq) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq) + H_2(g)\]. Magnesium hydroxide (milk of magnesia), sulfate (Epsom salts), chloride and citrate are all used in medicine. C Magnesium-25 If the percent abundance of magnesium-25 is Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. These are used in the production of many other kinds of organic and organometallic compounds.
Chemical element, metallic, symbol Mg, situated in group IIa in the periodic table, atomic number: 12, atomic weight: 24,312. Magnesium is commercially produced by electrolysis of molten magnesium chloride (MgCl2), processed mainly from seawater and by the direct reduction of its compounds with suitable reducing agentse.g., from the reaction of magnesium oxide or calcined dolomite with ferrosilicon (the Pidgeon process). It is given by the ratio of the pressure on a body to the fractional decrease in volume. The symbol for an atom indicates the element by its common two-letter symbol. abundance 78.99%) 25 Mg (isotypic mass 24.9858 amu, abundance 10.00%), and 26 Because the masses of all other atoms are calculated relative to the 12C standard, 12C is the only atomwhose exact atomic mass is equal to the mass number. Data for this section been provided by the British Geological Survey. The masses of the first two isotopes and percent abundances are as follows: 24.1687 amu, 78.900% 25.4830 amu, Magnesium is used in products that benefit from being lightweight, such as car seats, luggage, laptops, cameras and power tools. This acid is found naturally in citrus fruits and gives them their tart, Magnesium takes it name from magnesite ore, named for the district Magnesia in Thessaly, Greece. These alloys are useful in aeroplane and car construction. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Magnesium is a strong metal that is light and silvery-white.