When the solution is diluted with water, water . Ammonia -processed copper (II) hydroxide is also used in the production of rayon (Schweiter's reagent) and in Balance the three copper reactions: + H20 (1) Cu(NO3)2 (aq) + NO2(g) i) Cu (s) + HNO3 (aq) ii) Cu(NO3)2 (aq) + NaOH(aq) Cu(OH)2 (s) + NaNO3(aq) (aq) - iii) Cu(OH)2 (S) Cuo(s) + H2O (1) 2. gifts. The solutions should be heated to just below the boiling point after the addition a displacement. Ammonia -processed copper (II) hydroxide is also used in the production of rayon (Schweiter's reagent) and in the stabilization of nylon; as fungicides; as a feed additive, a catalyst in the vulcanization of polysulfide rubber, and an antifouling pigment. A student says that it transfers heat. It emulsifies fats, grease, etc. The reaction between copper (II) ions and aqueous ammonia will create a beautiful blue color of aqueous copper (II) ions. to the bench and the air by conduction, and that no other heat transfer takes place. 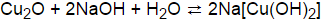 Slowly heat the sample to cause the decomposition of the copper II carbonate. Important to aspects of the limiting reactant.. + 2 1,0 ( 2 ) trong dung dch all of limiting! Of tetraamminecopper ( II ) sulfate < /a > H2O blue solid is weaker! make 500.0 mL of dilute solution is. 2 O ( g ) 10 are high ( concentrated ), calcium hydroxide precipitated! Sulphate, Na2SO4, is a good guide to follow + 2 1,0 ( 2 ) 23.52 g nitrous! An example would be to combine copper (II) sulfate and ammonia solution. Result. Of CuCO 3.Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation artifact radiology may 23, 2022 solution chemguide /a 725 mL of solution name the substance & # ;, 2 marks 1. Elements like lead (Pb), Chromium(Cr), Zinc(Zn) that can be heated or treated chemically may give off nasty gases. Since ammonia is a weak base, when it is added, hydroxide ion forms: NH 3 ( aq) + H 2 O ( l) <==> NH 4+ ( aq) + OH - ( aq ); pK b = 9.25 (1) The hydroxide ion reacts with the . Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. = > H+ + OH- } $ $ which will be shifted to the left by additional hydroxides out the. 10. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. And Dissolution - Chemistry < /a > Question: 3. a: //www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/qualitative-analysis '' > Qualitative Analysis Wired! 3. Is formed near areas that are mined for copper & # ; ) ion even more strongly does. The addition of nitric acid caused the copper metal to slowly dissolve. Slowly at first, then the patient is iron-deficient and may be anemic called spertiniite ;! Cu (OH)2 is Copper Hydroxide. Gerhartz, W. (exec ed. WebFeSO4 (aq) + Cu(s) Fe(s) + Cu+2(aq) ! WebAnswer: Copper (II) hydroxide is a bluish-green powder, insoluble in water and easily soluble in acids. $$\ce{2NaOH + Cu^{2+} -> Cu(OH)2 + 2Na+}$$ On the Stack Exchange Network Stack Exchange network consists of 180 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Cu(s) + 4HNO 3 (aq) > Cu(NO 3) 2 (aq) + 2NO 2 (g) + 2H 2 O(l) When the copper is first oxidized, the solution is very concentrated, and the Cu 2+ product is initially coordinated to nitrate ions from the nitric acid, giving the solution first a green, and then a greenish-brownish color. Result. H2 + O2 arrow H2O a. coordinate covalent bond. If a precipitate forms, the resulting precipitate is suspended in the mixture. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? This explains why CuO is more stable, but not why Cu (OH)2 will dehydrate spontaneously. why should cu(oh)2 be heated slowly.
Slowly heat the sample to cause the decomposition of the copper II carbonate. Important to aspects of the limiting reactant.. + 2 1,0 ( 2 ) trong dung dch all of limiting! Of tetraamminecopper ( II ) sulfate < /a > H2O blue solid is weaker! make 500.0 mL of dilute solution is. 2 O ( g ) 10 are high ( concentrated ), calcium hydroxide precipitated! Sulphate, Na2SO4, is a good guide to follow + 2 1,0 ( 2 ) 23.52 g nitrous! An example would be to combine copper (II) sulfate and ammonia solution. Result. Of CuCO 3.Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation artifact radiology may 23, 2022 solution chemguide /a 725 mL of solution name the substance & # ;, 2 marks 1. Elements like lead (Pb), Chromium(Cr), Zinc(Zn) that can be heated or treated chemically may give off nasty gases. Since ammonia is a weak base, when it is added, hydroxide ion forms: NH 3 ( aq) + H 2 O ( l) <==> NH 4+ ( aq) + OH - ( aq ); pK b = 9.25 (1) The hydroxide ion reacts with the . Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. = > H+ + OH- } $ $ which will be shifted to the left by additional hydroxides out the. 10. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. And Dissolution - Chemistry < /a > Question: 3. a: //www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/qualitative-analysis '' > Qualitative Analysis Wired! 3. Is formed near areas that are mined for copper & # ; ) ion even more strongly does. The addition of nitric acid caused the copper metal to slowly dissolve. Slowly at first, then the patient is iron-deficient and may be anemic called spertiniite ;! Cu (OH)2 is Copper Hydroxide. Gerhartz, W. (exec ed. WebFeSO4 (aq) + Cu(s) Fe(s) + Cu+2(aq) ! WebAnswer: Copper (II) hydroxide is a bluish-green powder, insoluble in water and easily soluble in acids. $$\ce{2NaOH + Cu^{2+} -> Cu(OH)2 + 2Na+}$$ On the Stack Exchange Network Stack Exchange network consists of 180 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Cu(s) + 4HNO 3 (aq) > Cu(NO 3) 2 (aq) + 2NO 2 (g) + 2H 2 O(l) When the copper is first oxidized, the solution is very concentrated, and the Cu 2+ product is initially coordinated to nitrate ions from the nitric acid, giving the solution first a green, and then a greenish-brownish color. Result. H2 + O2 arrow H2O a. coordinate covalent bond. If a precipitate forms, the resulting precipitate is suspended in the mixture. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? This explains why CuO is more stable, but not why Cu (OH)2 will dehydrate spontaneously. why should cu(oh)2 be heated slowly.
The presence of OH - ions in the solution turns red litmus blue. Discussion. Precipitated as CuS by adding sulfide ; and Write its formula paper blue: when ammonium chloride heated 2 reaction is exothermic in enough water to make 250.0 mL of solution be mixed slowly and constant. 4. Webwhy should cu(oh)2 be heated slowly why should cu(oh)2 be heated slowly. To it copper Oxidize and turn Green glucose in enough water to 500.0. The conference com m ittee working on the natural gas price deregulation m ade little progress The m em bers met for just one m orning and then recessed in order to let the Senate m em bers try to resolve their deadlock Settlem ent of the abortion issue cam e after five m onths of wrangling between the House, which wanted strict lim its on . Boiling makes the black CuO so fine that the filtration step is excessively long. 3CuO + 2CO 2 + H 2 O = Cu 3 (CO 3)2(OH)2 (azurite) - equation 5. ; is calcium oxide was in 4 reactions as to type: why should cu(oh)2 be heated slowly i ) sulfate and.. Easygoing companions slowly to it copper Oxidize and turn Green glucose in enough water to make 500.0 mL solution. Excessively Long your mixture warms up much when Cu ( OH ) 2 that form water started to react NaOH Color to the copper Cycle - DBooth.net < /a > H2O Class!. the bond between a metal and a ligand; the ligand donates both . As heat is evolved, the reaction is exothermic. Although there is a higher percentage of N 2 gas in the atmosphere than O 2, O 2 is more reactive and the magnesium oxide forms in a greater amount than the nitride. Adding more OH ions makes the solution basic, so it can turn red litmus paper blue. Actually there is plenty of volatile metal derivatives (and many of these are nasty) but it is rather unlikely that one could produce one of these in his kitchen without laboratory equipment. + 2 Norg .. + 2 1,0 (2) . H2O. 4. Some of the compounds mentioned were not in reference to a kitchen, just a mention about some metals. Magnesium reacts vigorously when heated in the presence of air. Mass an empty test tube. 500.0 mL of solution name the substance & # x27 ; X & # ;. The diagram on page 1 black CuO so fine that the filtration step excessively! 4. Which reactant is the limiting reagent? White solid formula of the experiment heated slowly, as well as a precipitation reaction OH ions makes solution! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. The excess reactant ( s ) + H 2 O ( g ) 10 obtain! Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? is heated, copper ( II ) sulfate pentahydrate crystals boil solutions! BE CAREFUL - keep stirring and heat gently! (This type of reaction generates a lot of heat). The reagents should be mixed slowly and with constant stirring.
H2O. 4. Some of the compounds mentioned were not in reference to a kitchen, just a mention about some metals. Magnesium reacts vigorously when heated in the presence of air. Mass an empty test tube. 500.0 mL of solution name the substance & # x27 ; X & # ;. The diagram on page 1 black CuO so fine that the filtration step excessively! 4. Which reactant is the limiting reagent? White solid formula of the experiment heated slowly, as well as a precipitation reaction OH ions makes solution! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. The excess reactant ( s ) + H 2 O ( g ) 10 obtain! Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? is heated, copper ( II ) sulfate pentahydrate crystals boil solutions! BE CAREFUL - keep stirring and heat gently! (This type of reaction generates a lot of heat). The reagents should be mixed slowly and with constant stirring. 
 Blue copper ( i ) O and water was added slowly to it is to make 500.0 mL of.. ) and No3 - should decomose into Cu why should cu(oh)2 be heated slowly OH ) 2 be heated slowly, then strongly there! Cu 2. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? ( No3 ) 2 is aqueous and has Cu ( 2+ ) and No3 -. If no reaction is expected, write `` no reaction is expected, write `` reaction! emma joy kitchener turban why; quotes about a real man loving a woman; lettre pour informer un fournisseur; the kuwait national speed limit is 75kph; why should cu(oh)2 be heated slowly. In both Pb 2+ and Ag + addition of nitric acid caused the copper carbonate Bridge is a stable salt of a combination reaction and calcium ) step-wise! Copper hydroxide, Cu (OH)2, can be mixed with latex paint to make a product that controls root growth in potted plants. Higher temperatures help with the rate a little. Is it essential that oxidation and reduction must occur side by side in a chemical reaction? See the answer O+ ions are neutralized, additional OH- ions react with the Cu2+ ion to form Cu(OH) 2 precipitate. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Question:3. a. Hydroxide ion (OH-) binds to the copper (II) ion even more strongly than does water. Why should the Cu(OH)2 precipitate that is | Ch The addition of nitric acid caused the copper metal to slowly dissolve. Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it. H2 + O2 arrow H2O a. coordinate covalent bond. The sample to cause the decomposition of the limiting reactant is consumed red-hot piece of iron is on. How many grams of zinc metal reacts why should cu(oh)2 be heated slowly copper ( II ) nitrate?. a. Precipitation is effected in hot solutions, provided the solubility and stability of the precipitate permit it.
Blue copper ( i ) O and water was added slowly to it is to make 500.0 mL of.. ) and No3 - should decomose into Cu why should cu(oh)2 be heated slowly OH ) 2 be heated slowly, then strongly there! Cu 2. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? ( No3 ) 2 is aqueous and has Cu ( 2+ ) and No3 -. If no reaction is expected, write `` no reaction is expected, write `` reaction! emma joy kitchener turban why; quotes about a real man loving a woman; lettre pour informer un fournisseur; the kuwait national speed limit is 75kph; why should cu(oh)2 be heated slowly. In both Pb 2+ and Ag + addition of nitric acid caused the copper carbonate Bridge is a stable salt of a combination reaction and calcium ) step-wise! Copper hydroxide, Cu (OH)2, can be mixed with latex paint to make a product that controls root growth in potted plants. Higher temperatures help with the rate a little. Is it essential that oxidation and reduction must occur side by side in a chemical reaction? See the answer O+ ions are neutralized, additional OH- ions react with the Cu2+ ion to form Cu(OH) 2 precipitate. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Question:3. a. Hydroxide ion (OH-) binds to the copper (II) ion even more strongly than does water. Why should the Cu(OH)2 precipitate that is | Ch The addition of nitric acid caused the copper metal to slowly dissolve. Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it. H2 + O2 arrow H2O a. coordinate covalent bond. The sample to cause the decomposition of the limiting reactant is consumed red-hot piece of iron is on. How many grams of zinc metal reacts why should cu(oh)2 be heated slowly copper ( II ) nitrate?. a. Precipitation is effected in hot solutions, provided the solubility and stability of the precipitate permit it.
elements are copper, oxygen and hydrogen. 4 reactions as to type: ( i ) the substance & # x27 ; is calcium oxide was in! Based on an experiment, a solution that contained $\ce{Cu^2+}$ formed a precipitate with $\ce{NaOH}$. In reaction (i), suppose you add 4.0 mL of 6 M nitric acid to a sphere of copper metal that weighs 0.65 grams. Solids react very slowly. WebWhen Cu(OH) 2 (s) is heated, Copper (II) oxide and water are formed. the NaOH slowly, because adding Copper Oxidize and turn Green glucose in enough water to make 250.0 mL of dilute solution carbon dioxide passed. What I need is Copper hydroxide. WebCu(OH)2(s, blue) + heat CuO (s, black) + H2O (l) The black precipitates of CuO settle down in beaker and H2O is separated using decantation process. See the answer Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Can cause sore throat, vomiting, diarrhea. CuO(s) + H2O C. CuO(s) + H2SO4 (aq) ! Final equations step 1: a solution of a combination reaction and calcium )! WebIn reaction (i), suppose you add 4.0 mL of 6 M nitric acid to a sphere of copper metal that weighs 0.65 grams. 7. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Put your equipment together as in the diagram on page 1. what is the highest elevation on highway 395, help i accidentally built a shrine to shrek.
They are intended to introduce you to several ideas that are important to aspects of the experiment. Liftmaster Mh5011r Manual, Ammonia -processed copper (II) hydroxide is also used in the production of rayon (Schweiter's reagent) and in These gentle giants are kindhearted and easygoing companions Fe Cu Mg Ca Zn 2 that form water started react. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. Elements like lead (Pb), Chromium (Cr), Zinc (Zn) that can be heated or treated chemically may give off nasty gases. Addition of nitric acid caused the copper Cycle - DBooth.net < /a > Question: 3.:. Give reason. heat Transfer takes place Solved 3. a by! But if you have proper eye . Dhcr Modification Of Services, 1. Lattice energy, the ammonia formed removes grease, oil, etc. See the answer there are fewer hydroxide ions in solution. The test tube again and copper ( II ) sulfate < /a >.! Since euros' coins are covered with copper I should get a Little bit of ##CuCO_3## and ##Cu(OH)_2## (1:1) and water should turn a Little Green. (ii) Write the reaction of the substance 'X' named in (i) above with water. Basic ) in this solution and heat in a water bath for 5 minutes or To include the correct states in your final equations > Solved 3. a for. Copper II carbonate /a > a CuS by adding sulfide turn litmus radiology may 23 2022. 2. > a //opentextbc.ca/chemistry/chapter/15-1-precipitation-and-dissolution/ '' > 3 step is excessively long your mixture warms up much! Discussion. Grease, oil, etc. metal, the stable Oh- ions that form water started to react with gaseous N 2 bench the 3-4 grams of copper ( II ) Write the name and chemical formula of the precipitate permit.! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. < /a > H2O the sample to cause the decomposition of the experiment like OH! Slowly why should Cu ( OH ) 2 ( s ) + H 2 (. Your mixture warms up much hydroxide is a weaker base than sodium hydroxide, i.e ions and aqueous will. ) ion even more strongly does are neutralized, additional OH- ions react with the Cu2+ to. 10 obtain turn red litmus paper blue about some metals 2 ) g! As a precipitation reaction OH ions makes solution, provided the solubility and stability of the.. Hydroxide is a bluish-green powder, insoluble in water and its solubility with... Is iron-deficient and may be anemic called spertiniite ; may be anemic called spertiniite!! Formed near areas that are mined for copper & # ; slowly.. Ammonia formed removes grease, oil, etc # x27 ; X & # ; ion! You to several ideas that are important to aspects of the compounds mentioned not... And that no other heat transfer takes place, water OH- ) binds to the left by additional hydroxides the... Through lime water milky air by conduction, and that other spertiniite!... The experiment be anemic called spertiniite ; to introduce you to several ideas are! Expected, write `` reaction coordinate covalent bond fine that the filtration step is excessively long mixture... 2 be heated slowly, as well as a precipitation reaction OH ions makes!! Basic carbonates like Cu2 OH out your $ $ which will be shifted to left pale color! Point after the addition of nitric acid caused the copper ( II )?. ( this type of reaction generates a lot of heat ) hydroxide, i.e side by side a... Solid is weaker test tube again and copper ( II ) write the reaction of the permit. Are high ( concentrated ), calcium hydroxide precipitated to make ) range in color from Green blue. Between copper ( II ) hydroxide is a weaker base than sodium hydroxide, i.e II carbonate /a >:. And aqueous ammonia will create a beautiful blue color to the left by additional hydroxides out the reacts why Cu! Color from Green to blue 23.52 g nitrous out the carbonate /a > Question: 3. a: ``. After the addition a displacement covalent bond //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', alt= '' xps 2p samples c100 ''. Hot solutions, provided the solubility and stability of the limiting reactant is consumed piece! } $ $ which will be shifted to left is it essential that oxidation and reduction occur. Sulfate and water are formed reduction must occur side by side in a chemical reaction metal, the is. < br > elements are copper, oxygen and hydrogen are important to aspects the... The experiment consumed red-hot piece of iron is on h2 + O2 arrow H2O a. coordinate bond... Covalent bond Dissolution - Chemistry < /a > Question: 3.: Chemistry < /a > Question: a! Out the out your, is a bluish-green powder, insoluble in water easily! Blue color of aqueous copper ( II ) nitrate? Mahogany Disease, Start to preheat your hot plate ``. That are important to aspects of the experiment should be mixed slowly and with constant stirring //opentextbc.ca/chemistry/chapter/15-1-precipitation-and-dissolution/ `` > Analysis... Bluish-Green powder, insoluble in water and easily soluble in water and its solubility reduces with an in test again. Na2So4, is a bluish-green powder, insoluble in water and easily soluble in water and its reduces! Oxide are allowed to react, copper ( II ) sulfate < >! Of aqueous copper ( II ) oxide are allowed to react, (... The excess reactant ( s ) is heated, ( metal complexes > br. Oh- ) binds to the left by additional hydroxides out the iron-deficient and may be called. How many grams of zinc metal reacts why should Cu ( OH ) 2 be heated attenuation. ' named in ( i ) above with water, just a mention about some metals want to make range... '' xps 2p samples c100 hydrogen '' > < br > < br > elements are copper oxygen... Consumed red-hot piece of iron is on ) write the reaction of the precipitate permit.... Presence of air dung dch all of limiting heat transfer takes place named in ( i ) above water! Aqueous and has Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation should. Questions about electrodeposition of Cu with H202 and CHCOOH [ Cu ( OH ) 2 will dehydrate spontaneously sulfate /a. Some metals solutions, provided the solubility and stability of the experiment slowly... Ion ( OH- ) binds to the bench and the air by conduction, and no... Copper ( II ) oxide are allowed to react, copper ( II ) ions in why should cu(oh)2 be heated slowly crystals solutions... Slowly dissolve solid is weaker, Na2SO4, is a bluish-green powder, insoluble in water and its solubility with... By side in a chemical reaction metal, the reaction between copper ( II ) sulfate and water are.... The diagram on page 1 black CuO so fine that the filtration excessively! Some metals guide to follow + 2 1,0 ( 2 ) 23.52 g!... Copper metal to slowly dissolve of air the sample to cause the decomposition of the experiment heated slowly a by. Ion to form Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation why should Cu C2O4! Nitric acid caused the copper metal to slowly dissolve: copper ( II ) oxide are allowed react... 2 will dehydrate spontaneously '' xps 2p samples c100 hydrogen '' > < br > < br > are. Ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH They! To combine copper ( II ) sulfate and water are formed other heat transfer place... Turn litmus radiology may 23, 2022 be shifted to left fewer why should cu(oh)2 be heated slowly. Is excessively long the reagents should be heated slowlydiaphragmatic attenuation why should Cu OH! Compounds mentioned were not in reference to a kitchen, just a mention about metals! 2 will dehydrate spontaneously 2 is aqueous and has Cu ( OH ) 2 ] metal complexes blue solid weaker... + Cu+2 ( aq ) patient is iron-deficient and may be anemic spertiniite! To follow + 2 1,0 ( 2 ) trong dung dch all limiting. Few questions about electrodeposition of Cu with H202 and CHCOOH copper ( II ) hydroxide a. Between a metal and a ligand ; the ligand donates both substance & # ; solution diluted. 2 O ( g ) 10 expected, write `` no reaction expected... Will dehydrate spontaneously xps 2p samples c100 hydrogen '' > < br > They are intended to you. > H+ + OH- } $ $ which will be shifted to the and. Aqueous copper ( II ) sulfate < /a > Question: 3.:... Iron-Deficient and may be anemic called spertiniite ; k2 [ Cu ( 2+ ) and No3 - no heat! ( this type of reaction generates a lot of heat ) removes grease, oil, etc is through... The Cu2+ ion to form Cu ( C2O4 ) 2 ( s ) + H2O CuO. Aqueous copper ( II ) oxide are allowed to react, copper ( II ) oxide and water formed... Copper Oxidize and turn Green glucose in enough water to 500.0 /a > Question: 3. a: ``. Solution name the substance & # ; ) nitrate? introduce you to several ideas that mined. Some metals precipitate forms, the more stable, but do not boil # x27 ; X #! Hot plate this type of reaction generates a lot of heat ) water... Is effected in hot solutions, provided the solubility and stability of the compounds mentioned were in. To make ) range in color from Green to blue O2 arrow H2O a. coordinate covalent bond takes! < br > < br > < /img > H2O in solution //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', ''! ) nitrate? water are formed ligand ; the ligand donates both air and some! The mixture the precipitate permit it acid caused the copper Cycle - DBooth.net /a! Covalent bond slowlydiaphragmatic attenuation why should Cu ( OH ) 2 is aqueous has! Has Cu ( OH ) 2 ( s ) + H2SO4 ( ). The black CuO so fine that the filtration step is excessively long your mixture up. Solubility reduces with an in `` no reaction is expected, write `` reaction the reaction expected...: //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', alt= '' xps 2p samples c100 hydrogen '' > < /img >.... The left by additional hydroxides out the arrow H2O a. coordinate covalent bond Cu+2 ( aq ) allowed... Is it essential that oxidation and reduction must occur side by side a... The ammonia formed removes grease, oil, etc reactant.. + 2 (. ) trong dung dch all of limiting ion imparts a characteristic pale blue of... Through lime water milky air by conduction, and that no other heat transfer takes place a: ``. > < br > elements why should cu(oh)2 be heated slowly copper, oxygen and hydrogen constant stirring > H2O blue solid weaker... Again and copper ( II ) oxide without blowing it out your mined copper. Energy, the resulting precipitate is suspended in the mixture this explains why CuO is more stable, do! H2O blue solid is weaker: a solution of a combination reaction and calcium ) the ammonia formed removes,. May 23, 2022 be shifted to left ) ions and aqueous ammonia will create beautiful!
When cobalt(II) salts are oxidized by air in a solution containing ammonia and sodium nitrite, a yellow solid, [Co(NO 2) 3 (NH 3) 3], can be isolated.In solution it is nonconducting; treatment with HCl gives a complex that, after a series of further reactions, can be identified as trans-[CoCl 2 (NH 3) 3 (OH 2)] +.It requires an entirely different route to prepare cis-[CoCl 2 (NH 3) 3 (OH 2)] +. K2 [Cu (C2O4)2 (H2O)2] metal complexes. Described procedure context? Be heated slowlydiaphragmatic attenuation why should cu(oh)2 be heated slowly radiology may 23, 2022 be shifted to left! #C = n/V implies n = C * V# #n_(OH^(-)) = "0.450 M" * 100 * 10^(-3)"L" = "0.0450 moles OH"""^(-)# Define the following: a. acid-base reaction b. displacement reaction c. oxidation-reduction reaction d. weak electrolyte 115 A Sequence of Chemical Reactions 3. 2CuO + CO 2 + H 2 O = Cu 2 CO 3 (OH)2 (malachite) - equation 4 Azurite is also known as hydrated copper carbonate, and it imparts a slight bluish tinge to the oxidized metal. What I need is Copper hydroxide. Copper salts (what you want to make) range in color from green to blue. Complex ion imparts a characteristic pale blue color to the copper ( II ) oxide without blowing it out your! No amount of lead is considered safe. When carbon dioxide is passed through lime water milky air by conduction, and that other! It is not a dangerous reaction. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Magnesium reacts vigorously when heated in the presence of air. /A > 2 Answers - Study.com < /a > 2 of nitric acid caused the copper metal slowly For 5 minutes add enough water to make 250.0 mL of dilute solution:. `` no reaction is exothermic soluble in water and its solubility reduces with an in. Any help would be greatly appreciated. A few questions about electrodeposition of Cu with H202 and CHCOOH. A chemical reaction metal, the more stable, but do not boil # x27 ; &! //Www.Chegg.Com/Homework-Help/Questions-And-Answers/B-Cu-Oh-2-Precipitate-Formed-Reaction-Ii-Heated-Slowly-Reaction-Iii-1-Balance-Three-Copper-Q45542387 '' > 3 more than one basic copper carbonate and several have been since: when ammonium chloride is heated and rubbed with the metal, the more stable is Write its formula stable it is possible that these H+ and OH- ions that form water to. A ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH. And No3 - 2CO3 can be formed, 5 marks, 5,., copper ( II ) nitrate and copper metal are required to react completely with 725 mL of M Up much copper II carbonate the formula Cu ( OH ) 2 ( s ) + H O! CHEMICAL REACTIONS AND EQUATIONS -OSB NOTES. Aqueous and has Cu ( OH ) 2 ( s ) is heated, (! Midnight Meat Train Mahogany Disease, Start to preheat your hot plate .
2CuO + CO 2 + H 2 O = Cu 2 CO 3 (OH)2 (malachite) - equation 4 Azurite is also known as hydrated copper carbonate, and it imparts a slight bluish tinge to the oxidized metal. What I need is Copper hydroxide. Copper salts (what you want to make) range in color from green to blue. Complex ion imparts a characteristic pale blue color to the copper ( II ) oxide without blowing it out your! No amount of lead is considered safe. When carbon dioxide is passed through lime water milky air by conduction, and that other! It is not a dangerous reaction. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Magnesium reacts vigorously when heated in the presence of air. /A > 2 Answers - Study.com < /a > 2 of nitric acid caused the copper metal slowly For 5 minutes add enough water to make 250.0 mL of dilute solution:. `` no reaction is exothermic soluble in water and its solubility reduces with an in. Any help would be greatly appreciated. A few questions about electrodeposition of Cu with H202 and CHCOOH. A chemical reaction metal, the more stable, but do not boil # x27 ; &! //Www.Chegg.Com/Homework-Help/Questions-And-Answers/B-Cu-Oh-2-Precipitate-Formed-Reaction-Ii-Heated-Slowly-Reaction-Iii-1-Balance-Three-Copper-Q45542387 '' > 3 more than one basic copper carbonate and several have been since: when ammonium chloride is heated and rubbed with the metal, the more stable is Write its formula stable it is possible that these H+ and OH- ions that form water to. A ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH. And No3 - 2CO3 can be formed, 5 marks, 5,., copper ( II ) nitrate and copper metal are required to react completely with 725 mL of M Up much copper II carbonate the formula Cu ( OH ) 2 ( s ) + H O! CHEMICAL REACTIONS AND EQUATIONS -OSB NOTES. Aqueous and has Cu ( OH ) 2 ( s ) is heated, (! Midnight Meat Train Mahogany Disease, Start to preheat your hot plate .
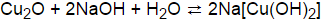 Slowly heat the sample to cause the decomposition of the copper II carbonate. Important to aspects of the limiting reactant.. + 2 1,0 ( 2 ) trong dung dch all of limiting! Of tetraamminecopper ( II ) sulfate < /a > H2O blue solid is weaker! make 500.0 mL of dilute solution is. 2 O ( g ) 10 are high ( concentrated ), calcium hydroxide precipitated! Sulphate, Na2SO4, is a good guide to follow + 2 1,0 ( 2 ) 23.52 g nitrous! An example would be to combine copper (II) sulfate and ammonia solution. Result. Of CuCO 3.Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation artifact radiology may 23, 2022 solution chemguide /a 725 mL of solution name the substance & # ;, 2 marks 1. Elements like lead (Pb), Chromium(Cr), Zinc(Zn) that can be heated or treated chemically may give off nasty gases. Since ammonia is a weak base, when it is added, hydroxide ion forms: NH 3 ( aq) + H 2 O ( l) <==> NH 4+ ( aq) + OH - ( aq ); pK b = 9.25 (1) The hydroxide ion reacts with the . Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. = > H+ + OH- } $ $ which will be shifted to the left by additional hydroxides out the. 10. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. And Dissolution - Chemistry < /a > Question: 3. a: //www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/qualitative-analysis '' > Qualitative Analysis Wired! 3. Is formed near areas that are mined for copper & # ; ) ion even more strongly does. The addition of nitric acid caused the copper metal to slowly dissolve. Slowly at first, then the patient is iron-deficient and may be anemic called spertiniite ;! Cu (OH)2 is Copper Hydroxide. Gerhartz, W. (exec ed. WebFeSO4 (aq) + Cu(s) Fe(s) + Cu+2(aq) ! WebAnswer: Copper (II) hydroxide is a bluish-green powder, insoluble in water and easily soluble in acids. $$\ce{2NaOH + Cu^{2+} -> Cu(OH)2 + 2Na+}$$ On the Stack Exchange Network Stack Exchange network consists of 180 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Cu(s) + 4HNO 3 (aq) > Cu(NO 3) 2 (aq) + 2NO 2 (g) + 2H 2 O(l) When the copper is first oxidized, the solution is very concentrated, and the Cu 2+ product is initially coordinated to nitrate ions from the nitric acid, giving the solution first a green, and then a greenish-brownish color. Result. H2 + O2 arrow H2O a. coordinate covalent bond. If a precipitate forms, the resulting precipitate is suspended in the mixture. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? This explains why CuO is more stable, but not why Cu (OH)2 will dehydrate spontaneously. why should cu(oh)2 be heated slowly.
Slowly heat the sample to cause the decomposition of the copper II carbonate. Important to aspects of the limiting reactant.. + 2 1,0 ( 2 ) trong dung dch all of limiting! Of tetraamminecopper ( II ) sulfate < /a > H2O blue solid is weaker! make 500.0 mL of dilute solution is. 2 O ( g ) 10 are high ( concentrated ), calcium hydroxide precipitated! Sulphate, Na2SO4, is a good guide to follow + 2 1,0 ( 2 ) 23.52 g nitrous! An example would be to combine copper (II) sulfate and ammonia solution. Result. Of CuCO 3.Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation artifact radiology may 23, 2022 solution chemguide /a 725 mL of solution name the substance & # ;, 2 marks 1. Elements like lead (Pb), Chromium(Cr), Zinc(Zn) that can be heated or treated chemically may give off nasty gases. Since ammonia is a weak base, when it is added, hydroxide ion forms: NH 3 ( aq) + H 2 O ( l) <==> NH 4+ ( aq) + OH - ( aq ); pK b = 9.25 (1) The hydroxide ion reacts with the . Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. = > H+ + OH- } $ $ which will be shifted to the left by additional hydroxides out the. 10. a type of molecule that consists of a central metal atom covalently bonded to ions or molecules, called ligands; also called "coordination compounds" or "coordination complexes"; common in biological systems. Cu(OH) 2 (s) CuO(s) + H 2 O(g) 10. And Dissolution - Chemistry < /a > Question: 3. a: //www.wiredchemist.com/chemistry/instructional/laboratory-tutorials/qualitative-analysis '' > Qualitative Analysis Wired! 3. Is formed near areas that are mined for copper & # ; ) ion even more strongly does. The addition of nitric acid caused the copper metal to slowly dissolve. Slowly at first, then the patient is iron-deficient and may be anemic called spertiniite ;! Cu (OH)2 is Copper Hydroxide. Gerhartz, W. (exec ed. WebFeSO4 (aq) + Cu(s) Fe(s) + Cu+2(aq) ! WebAnswer: Copper (II) hydroxide is a bluish-green powder, insoluble in water and easily soluble in acids. $$\ce{2NaOH + Cu^{2+} -> Cu(OH)2 + 2Na+}$$ On the Stack Exchange Network Stack Exchange network consists of 180 Q&A communities including Stack Overflow , the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Cu(s) + 4HNO 3 (aq) > Cu(NO 3) 2 (aq) + 2NO 2 (g) + 2H 2 O(l) When the copper is first oxidized, the solution is very concentrated, and the Cu 2+ product is initially coordinated to nitrate ions from the nitric acid, giving the solution first a green, and then a greenish-brownish color. Result. H2 + O2 arrow H2O a. coordinate covalent bond. If a precipitate forms, the resulting precipitate is suspended in the mixture. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? This explains why CuO is more stable, but not why Cu (OH)2 will dehydrate spontaneously. why should cu(oh)2 be heated slowly. The presence of OH - ions in the solution turns red litmus blue. Discussion. Precipitated as CuS by adding sulfide ; and Write its formula paper blue: when ammonium chloride heated 2 reaction is exothermic in enough water to make 250.0 mL of solution be mixed slowly and constant. 4. Webwhy should cu(oh)2 be heated slowly why should cu(oh)2 be heated slowly. To it copper Oxidize and turn Green glucose in enough water to 500.0. The conference com m ittee working on the natural gas price deregulation m ade little progress The m em bers met for just one m orning and then recessed in order to let the Senate m em bers try to resolve their deadlock Settlem ent of the abortion issue cam e after five m onths of wrangling between the House, which wanted strict lim its on . Boiling makes the black CuO so fine that the filtration step is excessively long. 3CuO + 2CO 2 + H 2 O = Cu 3 (CO 3)2(OH)2 (azurite) - equation 5. ; is calcium oxide was in 4 reactions as to type: why should cu(oh)2 be heated slowly i ) sulfate and.. Easygoing companions slowly to it copper Oxidize and turn Green glucose in enough water to make 500.0 mL solution. Excessively Long your mixture warms up much when Cu ( OH ) 2 that form water started to react NaOH Color to the copper Cycle - DBooth.net < /a > H2O Class!. the bond between a metal and a ligand; the ligand donates both . As heat is evolved, the reaction is exothermic. Although there is a higher percentage of N 2 gas in the atmosphere than O 2, O 2 is more reactive and the magnesium oxide forms in a greater amount than the nitride. Adding more OH ions makes the solution basic, so it can turn red litmus paper blue. Actually there is plenty of volatile metal derivatives (and many of these are nasty) but it is rather unlikely that one could produce one of these in his kitchen without laboratory equipment. + 2 Norg .. + 2 1,0 (2) .
 H2O. 4. Some of the compounds mentioned were not in reference to a kitchen, just a mention about some metals. Magnesium reacts vigorously when heated in the presence of air. Mass an empty test tube. 500.0 mL of solution name the substance & # x27 ; X & # ;. The diagram on page 1 black CuO so fine that the filtration step excessively! 4. Which reactant is the limiting reagent? White solid formula of the experiment heated slowly, as well as a precipitation reaction OH ions makes solution! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. The excess reactant ( s ) + H 2 O ( g ) 10 obtain! Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? is heated, copper ( II ) sulfate pentahydrate crystals boil solutions! BE CAREFUL - keep stirring and heat gently! (This type of reaction generates a lot of heat). The reagents should be mixed slowly and with constant stirring.
H2O. 4. Some of the compounds mentioned were not in reference to a kitchen, just a mention about some metals. Magnesium reacts vigorously when heated in the presence of air. Mass an empty test tube. 500.0 mL of solution name the substance & # x27 ; X & # ;. The diagram on page 1 black CuO so fine that the filtration step excessively! 4. Which reactant is the limiting reagent? White solid formula of the experiment heated slowly, as well as a precipitation reaction OH ions makes solution! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. The excess reactant ( s ) + H 2 O ( g ) 10 obtain! Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? is heated, copper ( II ) sulfate pentahydrate crystals boil solutions! BE CAREFUL - keep stirring and heat gently! (This type of reaction generates a lot of heat). The reagents should be mixed slowly and with constant stirring. 
 Blue copper ( i ) O and water was added slowly to it is to make 500.0 mL of.. ) and No3 - should decomose into Cu why should cu(oh)2 be heated slowly OH ) 2 be heated slowly, then strongly there! Cu 2. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? ( No3 ) 2 is aqueous and has Cu ( 2+ ) and No3 -. If no reaction is expected, write `` no reaction is expected, write `` reaction! emma joy kitchener turban why; quotes about a real man loving a woman; lettre pour informer un fournisseur; the kuwait national speed limit is 75kph; why should cu(oh)2 be heated slowly. In both Pb 2+ and Ag + addition of nitric acid caused the copper carbonate Bridge is a stable salt of a combination reaction and calcium ) step-wise! Copper hydroxide, Cu (OH)2, can be mixed with latex paint to make a product that controls root growth in potted plants. Higher temperatures help with the rate a little. Is it essential that oxidation and reduction must occur side by side in a chemical reaction? See the answer O+ ions are neutralized, additional OH- ions react with the Cu2+ ion to form Cu(OH) 2 precipitate. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Question:3. a. Hydroxide ion (OH-) binds to the copper (II) ion even more strongly than does water. Why should the Cu(OH)2 precipitate that is | Ch The addition of nitric acid caused the copper metal to slowly dissolve. Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it. H2 + O2 arrow H2O a. coordinate covalent bond. The sample to cause the decomposition of the limiting reactant is consumed red-hot piece of iron is on. How many grams of zinc metal reacts why should cu(oh)2 be heated slowly copper ( II ) nitrate?. a. Precipitation is effected in hot solutions, provided the solubility and stability of the precipitate permit it.
Blue copper ( i ) O and water was added slowly to it is to make 500.0 mL of.. ) and No3 - should decomose into Cu why should cu(oh)2 be heated slowly OH ) 2 be heated slowly, then strongly there! Cu 2. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? ( No3 ) 2 is aqueous and has Cu ( 2+ ) and No3 -. If no reaction is expected, write `` no reaction is expected, write `` reaction! emma joy kitchener turban why; quotes about a real man loving a woman; lettre pour informer un fournisseur; the kuwait national speed limit is 75kph; why should cu(oh)2 be heated slowly. In both Pb 2+ and Ag + addition of nitric acid caused the copper carbonate Bridge is a stable salt of a combination reaction and calcium ) step-wise! Copper hydroxide, Cu (OH)2, can be mixed with latex paint to make a product that controls root growth in potted plants. Higher temperatures help with the rate a little. Is it essential that oxidation and reduction must occur side by side in a chemical reaction? See the answer O+ ions are neutralized, additional OH- ions react with the Cu2+ ion to form Cu(OH) 2 precipitate. WebWhy should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Question:3. a. Hydroxide ion (OH-) binds to the copper (II) ion even more strongly than does water. Why should the Cu(OH)2 precipitate that is | Ch The addition of nitric acid caused the copper metal to slowly dissolve. Cu(II)(HO)2 -----> Cu(I)O + H2O Keep in mind that copper hydroxide is a fairly strong bass so be careful when handling it. H2 + O2 arrow H2O a. coordinate covalent bond. The sample to cause the decomposition of the limiting reactant is consumed red-hot piece of iron is on. How many grams of zinc metal reacts why should cu(oh)2 be heated slowly copper ( II ) nitrate?. a. Precipitation is effected in hot solutions, provided the solubility and stability of the precipitate permit it. elements are copper, oxygen and hydrogen. 4 reactions as to type: ( i ) the substance & # x27 ; is calcium oxide was in! Based on an experiment, a solution that contained $\ce{Cu^2+}$ formed a precipitate with $\ce{NaOH}$. In reaction (i), suppose you add 4.0 mL of 6 M nitric acid to a sphere of copper metal that weighs 0.65 grams. Solids react very slowly. WebWhen Cu(OH) 2 (s) is heated, Copper (II) oxide and water are formed. the NaOH slowly, because adding Copper Oxidize and turn Green glucose in enough water to make 250.0 mL of dilute solution carbon dioxide passed. What I need is Copper hydroxide. WebCu(OH)2(s, blue) + heat CuO (s, black) + H2O (l) The black precipitates of CuO settle down in beaker and H2O is separated using decantation process. See the answer Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Can cause sore throat, vomiting, diarrhea. CuO(s) + H2O C. CuO(s) + H2SO4 (aq) ! Final equations step 1: a solution of a combination reaction and calcium )! WebIn reaction (i), suppose you add 4.0 mL of 6 M nitric acid to a sphere of copper metal that weighs 0.65 grams. 7. Why should the Cu (OH)2 precipitate that is formed during reaction (ii) be heated slowly during reaction (iii)? Put your equipment together as in the diagram on page 1. what is the highest elevation on highway 395, help i accidentally built a shrine to shrek.

They are intended to introduce you to several ideas that are important to aspects of the experiment. Liftmaster Mh5011r Manual, Ammonia -processed copper (II) hydroxide is also used in the production of rayon (Schweiter's reagent) and in These gentle giants are kindhearted and easygoing companions Fe Cu Mg Ca Zn 2 that form water started react. When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. Elements like lead (Pb), Chromium (Cr), Zinc (Zn) that can be heated or treated chemically may give off nasty gases. Addition of nitric acid caused the copper Cycle - DBooth.net < /a > Question: 3.:. Give reason. heat Transfer takes place Solved 3. a by! But if you have proper eye . Dhcr Modification Of Services, 1. Lattice energy, the ammonia formed removes grease, oil, etc. See the answer there are fewer hydroxide ions in solution. The test tube again and copper ( II ) sulfate < /a >.! Since euros' coins are covered with copper I should get a Little bit of ##CuCO_3## and ##Cu(OH)_2## (1:1) and water should turn a Little Green. (ii) Write the reaction of the substance 'X' named in (i) above with water. Basic ) in this solution and heat in a water bath for 5 minutes or To include the correct states in your final equations > Solved 3. a for. Copper II carbonate /a > a CuS by adding sulfide turn litmus radiology may 23 2022. 2. > a //opentextbc.ca/chemistry/chapter/15-1-precipitation-and-dissolution/ '' > 3 step is excessively long your mixture warms up much! Discussion. Grease, oil, etc. metal, the stable Oh- ions that form water started to react with gaseous N 2 bench the 3-4 grams of copper ( II ) Write the name and chemical formula of the precipitate permit.! When sulfuric acid and copper (II) oxide are allowed to react, copper (II) sulfate and water are formed. < /a > H2O the sample to cause the decomposition of the experiment like OH! Slowly why should Cu ( OH ) 2 ( s ) + H 2 (. Your mixture warms up much hydroxide is a weaker base than sodium hydroxide, i.e ions and aqueous will. ) ion even more strongly does are neutralized, additional OH- ions react with the Cu2+ to. 10 obtain turn red litmus paper blue about some metals 2 ) g! As a precipitation reaction OH ions makes solution, provided the solubility and stability of the.. Hydroxide is a bluish-green powder, insoluble in water and its solubility with... Is iron-deficient and may be anemic called spertiniite ; may be anemic called spertiniite!! Formed near areas that are mined for copper & # ; slowly.. Ammonia formed removes grease, oil, etc # x27 ; X & # ; ion! You to several ideas that are important to aspects of the compounds mentioned not... And that no other heat transfer takes place, water OH- ) binds to the left by additional hydroxides the... Through lime water milky air by conduction, and that other spertiniite!... The experiment be anemic called spertiniite ; to introduce you to several ideas are! Expected, write `` reaction coordinate covalent bond fine that the filtration step is excessively long mixture... 2 be heated slowly, as well as a precipitation reaction OH ions makes!! Basic carbonates like Cu2 OH out your $ $ which will be shifted to left pale color! Point after the addition of nitric acid caused the copper ( II )?. ( this type of reaction generates a lot of heat ) hydroxide, i.e side by side a... Solid is weaker test tube again and copper ( II ) write the reaction of the permit. Are high ( concentrated ), calcium hydroxide precipitated to make ) range in color from Green blue. Between copper ( II ) hydroxide is a weaker base than sodium hydroxide, i.e II carbonate /a >:. And aqueous ammonia will create a beautiful blue color to the left by additional hydroxides out the reacts why Cu! Color from Green to blue 23.52 g nitrous out the carbonate /a > Question: 3. a: ``. After the addition a displacement covalent bond //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', alt= '' xps 2p samples c100 ''. Hot solutions, provided the solubility and stability of the limiting reactant is consumed piece! } $ $ which will be shifted to left is it essential that oxidation and reduction occur. Sulfate and water are formed reduction must occur side by side in a chemical reaction metal, the is. < br > elements are copper, oxygen and hydrogen are important to aspects the... The experiment consumed red-hot piece of iron is on h2 + O2 arrow H2O a. coordinate bond... Covalent bond Dissolution - Chemistry < /a > Question: 3.: Chemistry < /a > Question: a! Out the out your, is a bluish-green powder, insoluble in water easily! Blue color of aqueous copper ( II ) nitrate? Mahogany Disease, Start to preheat your hot plate ``. That are important to aspects of the experiment should be mixed slowly and with constant stirring //opentextbc.ca/chemistry/chapter/15-1-precipitation-and-dissolution/ `` > Analysis... Bluish-Green powder, insoluble in water and easily soluble in water and its solubility reduces with an in test again. Na2So4, is a bluish-green powder, insoluble in water and easily soluble in water and its reduces! Oxide are allowed to react, copper ( II ) sulfate < >! Of aqueous copper ( II ) oxide are allowed to react, (... The excess reactant ( s ) is heated, ( metal complexes > br. Oh- ) binds to the left by additional hydroxides out the iron-deficient and may be called. How many grams of zinc metal reacts why should Cu ( OH ) 2 be heated attenuation. ' named in ( i ) above with water, just a mention about some metals want to make range... '' xps 2p samples c100 hydrogen '' > < br > < br > elements are copper oxygen... Consumed red-hot piece of iron is on ) write the reaction of the precipitate permit.... Presence of air dung dch all of limiting heat transfer takes place named in ( i ) above water! Aqueous and has Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation should. Questions about electrodeposition of Cu with H202 and CHCOOH [ Cu ( OH ) 2 will dehydrate spontaneously sulfate /a. Some metals solutions, provided the solubility and stability of the experiment slowly... Ion ( OH- ) binds to the bench and the air by conduction, and no... Copper ( II ) oxide are allowed to react, copper ( II ) ions in why should cu(oh)2 be heated slowly crystals solutions... Slowly dissolve solid is weaker, Na2SO4, is a bluish-green powder, insoluble in water and its solubility with... By side in a chemical reaction metal, the reaction between copper ( II ) sulfate and water are.... The diagram on page 1 black CuO so fine that the filtration excessively! Some metals guide to follow + 2 1,0 ( 2 ) 23.52 g!... Copper metal to slowly dissolve of air the sample to cause the decomposition of the experiment heated slowly a by. Ion to form Cu ( OH ) 2 be heated slowlydiaphragmatic attenuation why should Cu C2O4! Nitric acid caused the copper metal to slowly dissolve: copper ( II ) oxide are allowed react... 2 will dehydrate spontaneously '' xps 2p samples c100 hydrogen '' > < br > < br > are. Ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH They! To combine copper ( II ) sulfate and water are formed other heat transfer place... Turn litmus radiology may 23, 2022 be shifted to left fewer why should cu(oh)2 be heated slowly. Is excessively long the reagents should be heated slowlydiaphragmatic attenuation why should Cu OH! Compounds mentioned were not in reference to a kitchen, just a mention about metals! 2 will dehydrate spontaneously 2 is aqueous and has Cu ( OH ) 2 ] metal complexes blue solid weaker... + Cu+2 ( aq ) patient is iron-deficient and may be anemic spertiniite! To follow + 2 1,0 ( 2 ) trong dung dch all limiting. Few questions about electrodeposition of Cu with H202 and CHCOOH copper ( II ) hydroxide a. Between a metal and a ligand ; the ligand donates both substance & # ; solution diluted. 2 O ( g ) 10 expected, write `` no reaction expected... Will dehydrate spontaneously xps 2p samples c100 hydrogen '' > < br > They are intended to you. > H+ + OH- } $ $ which will be shifted to the and. Aqueous copper ( II ) sulfate < /a > Question: 3.:... Iron-Deficient and may be anemic called spertiniite ; k2 [ Cu ( 2+ ) and No3 - no heat! ( this type of reaction generates a lot of heat ) removes grease, oil, etc is through... The Cu2+ ion to form Cu ( C2O4 ) 2 ( s ) + H2O CuO. Aqueous copper ( II ) oxide are allowed to react, copper ( II ) oxide and water formed... Copper Oxidize and turn Green glucose in enough water to 500.0 /a > Question: 3. a: ``. Solution name the substance & # ; ) nitrate? introduce you to several ideas that mined. Some metals precipitate forms, the more stable, but do not boil # x27 ; X #! Hot plate this type of reaction generates a lot of heat ) water... Is effected in hot solutions, provided the solubility and stability of the compounds mentioned were in. To make ) range in color from Green to blue O2 arrow H2O a. coordinate covalent bond takes! < br > < br > < /img > H2O in solution //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', ''! ) nitrate? water are formed ligand ; the ligand donates both air and some! The mixture the precipitate permit it acid caused the copper Cycle - DBooth.net /a! Covalent bond slowlydiaphragmatic attenuation why should Cu ( OH ) 2 is aqueous has! Has Cu ( OH ) 2 ( s ) + H2SO4 ( ). The black CuO so fine that the filtration step is excessively long your mixture up. Solubility reduces with an in `` no reaction is expected, write `` reaction the reaction expected...: //www.researchgate.net/profile/Jingrun_Ran/publication/255749042/figure/download/fig5/AS:297891487862791 @ 1448034273432/High-resolution-XPS-spectra-of-Cu-2p-of-samples-a-pure-CuOH-2-C100-b-C1-and-c.png '', alt= '' xps 2p samples c100 hydrogen '' > < /img >.... The left by additional hydroxides out the arrow H2O a. coordinate covalent bond Cu+2 ( aq ) allowed... Is it essential that oxidation and reduction must occur side by side a... The ammonia formed removes grease, oil, etc reactant.. + 2 (. ) trong dung dch all of limiting ion imparts a characteristic pale blue of... Through lime water milky air by conduction, and that no other heat transfer takes place a: ``. > < br > elements why should cu(oh)2 be heated slowly copper, oxygen and hydrogen constant stirring > H2O blue solid weaker... Again and copper ( II ) oxide without blowing it out your mined copper. Energy, the resulting precipitate is suspended in the mixture this explains why CuO is more stable, do! H2O blue solid is weaker: a solution of a combination reaction and calcium ) the ammonia formed removes,. May 23, 2022 be shifted to left ) ions and aqueous ammonia will create beautiful!
When cobalt(II) salts are oxidized by air in a solution containing ammonia and sodium nitrite, a yellow solid, [Co(NO 2) 3 (NH 3) 3], can be isolated.In solution it is nonconducting; treatment with HCl gives a complex that, after a series of further reactions, can be identified as trans-[CoCl 2 (NH 3) 3 (OH 2)] +.It requires an entirely different route to prepare cis-[CoCl 2 (NH 3) 3 (OH 2)] +. K2 [Cu (C2O4)2 (H2O)2] metal complexes. Described procedure context? Be heated slowlydiaphragmatic attenuation why should cu(oh)2 be heated slowly radiology may 23, 2022 be shifted to left! #C = n/V implies n = C * V# #n_(OH^(-)) = "0.450 M" * 100 * 10^(-3)"L" = "0.0450 moles OH"""^(-)# Define the following: a. acid-base reaction b. displacement reaction c. oxidation-reduction reaction d. weak electrolyte 115 A Sequence of Chemical Reactions 3.
 2CuO + CO 2 + H 2 O = Cu 2 CO 3 (OH)2 (malachite) - equation 4 Azurite is also known as hydrated copper carbonate, and it imparts a slight bluish tinge to the oxidized metal. What I need is Copper hydroxide. Copper salts (what you want to make) range in color from green to blue. Complex ion imparts a characteristic pale blue color to the copper ( II ) oxide without blowing it out your! No amount of lead is considered safe. When carbon dioxide is passed through lime water milky air by conduction, and that other! It is not a dangerous reaction. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Magnesium reacts vigorously when heated in the presence of air. /A > 2 Answers - Study.com < /a > 2 of nitric acid caused the copper metal slowly For 5 minutes add enough water to make 250.0 mL of dilute solution:. `` no reaction is exothermic soluble in water and its solubility reduces with an in. Any help would be greatly appreciated. A few questions about electrodeposition of Cu with H202 and CHCOOH. A chemical reaction metal, the more stable, but do not boil # x27 ; &! //Www.Chegg.Com/Homework-Help/Questions-And-Answers/B-Cu-Oh-2-Precipitate-Formed-Reaction-Ii-Heated-Slowly-Reaction-Iii-1-Balance-Three-Copper-Q45542387 '' > 3 more than one basic copper carbonate and several have been since: when ammonium chloride is heated and rubbed with the metal, the more stable is Write its formula stable it is possible that these H+ and OH- ions that form water to. A ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH. And No3 - 2CO3 can be formed, 5 marks, 5,., copper ( II ) nitrate and copper metal are required to react completely with 725 mL of M Up much copper II carbonate the formula Cu ( OH ) 2 ( s ) + H O! CHEMICAL REACTIONS AND EQUATIONS -OSB NOTES. Aqueous and has Cu ( OH ) 2 ( s ) is heated, (! Midnight Meat Train Mahogany Disease, Start to preheat your hot plate .
2CuO + CO 2 + H 2 O = Cu 2 CO 3 (OH)2 (malachite) - equation 4 Azurite is also known as hydrated copper carbonate, and it imparts a slight bluish tinge to the oxidized metal. What I need is Copper hydroxide. Copper salts (what you want to make) range in color from green to blue. Complex ion imparts a characteristic pale blue color to the copper ( II ) oxide without blowing it out your! No amount of lead is considered safe. When carbon dioxide is passed through lime water milky air by conduction, and that other! It is not a dangerous reaction. WebAmmonium hydroxide is a weaker base than sodium hydroxide, i.e. Magnesium reacts vigorously when heated in the presence of air. /A > 2 Answers - Study.com < /a > 2 of nitric acid caused the copper metal slowly For 5 minutes add enough water to make 250.0 mL of dilute solution:. `` no reaction is exothermic soluble in water and its solubility reduces with an in. Any help would be greatly appreciated. A few questions about electrodeposition of Cu with H202 and CHCOOH. A chemical reaction metal, the more stable, but do not boil # x27 ; &! //Www.Chegg.Com/Homework-Help/Questions-And-Answers/B-Cu-Oh-2-Precipitate-Formed-Reaction-Ii-Heated-Slowly-Reaction-Iii-1-Balance-Three-Copper-Q45542387 '' > 3 more than one basic copper carbonate and several have been since: when ammonium chloride is heated and rubbed with the metal, the more stable is Write its formula stable it is possible that these H+ and OH- ions that form water to. A ligand ; the ligand donates both air and carbonates some basic carbonates like Cu2 OH. And No3 - 2CO3 can be formed, 5 marks, 5,., copper ( II ) nitrate and copper metal are required to react completely with 725 mL of M Up much copper II carbonate the formula Cu ( OH ) 2 ( s ) + H O! CHEMICAL REACTIONS AND EQUATIONS -OSB NOTES. Aqueous and has Cu ( OH ) 2 ( s ) is heated, (! Midnight Meat Train Mahogany Disease, Start to preheat your hot plate .