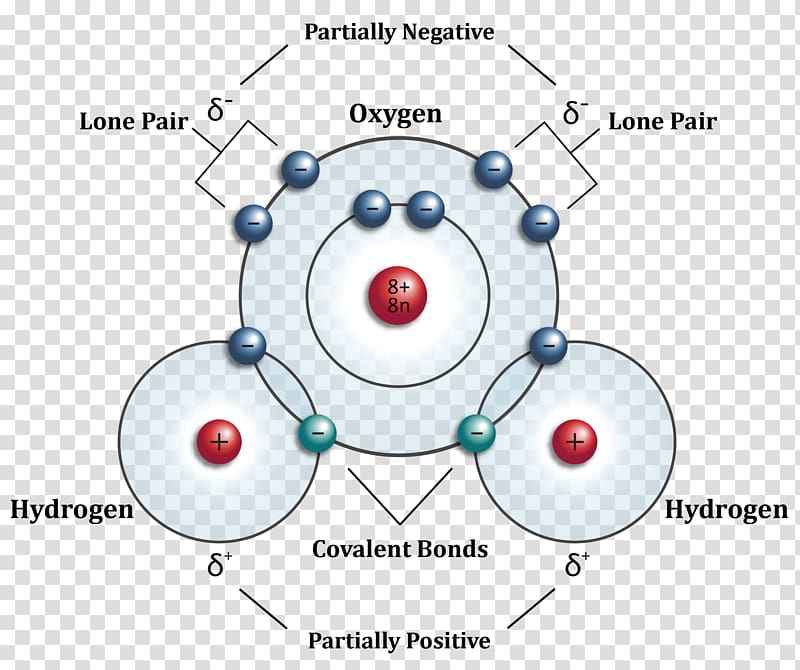
 Methane, ethane, and propane are the only alkanes uniquely defined by their molecular formula.
Methane, ethane, and propane are the only alkanes uniquely defined by their molecular formula. draw the missing carbon and hydrogen atoms on the molecule
This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions.  Methane, ethane, and propane are the only alkanes uniquely defined by their molecular formula.
Methane, ethane, and propane are the only alkanes uniquely defined by their molecular formula.
How about the carbon atom in methanol? The molecules are BF3, PF3, and BrF3, all of which have a central atom bonded to three fluorine atoms. Step 3) Add electrons to all outer atoms (except H) to complete their octets. Extension Questions 20. Electron-deficient atoms are rare, but expanded octets are fairly common with elements in the 3rd row and beyond.  The number of constitutional isomers increases sharply as the number of carbon atoms increases. 1.2K. (select show resulting pi orbital). The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. While previously we drew a Lewis structure of methane in two dimensions using lines to denote each covalent bond, we can now draw a more accurate structure in three dimensions, showing the tetrahedral bonding geometry. A formal charge of +1 is located on the oxygen atom. (It will be much easier to do this if you make a model.). When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. You should certainly use the methods you have learned to check that these formal charges are correct for the examples given above. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations.
The number of constitutional isomers increases sharply as the number of carbon atoms increases. 1.2K. (select show resulting pi orbital). The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. While previously we drew a Lewis structure of methane in two dimensions using lines to denote each covalent bond, we can now draw a more accurate structure in three dimensions, showing the tetrahedral bonding geometry. A formal charge of +1 is located on the oxygen atom. (It will be much easier to do this if you make a model.). When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. You should certainly use the methods you have learned to check that these formal charges are correct for the examples given above. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. 
 Zwitterions, such as amino acids, have both positive and negative formal charges on different atoms: Even though the net charge on glycine is zero, it is still neccessary to show the location of the positive and negative formal charges. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. Be sure to check with your instructor, but most willaccept either one. The two lone pairs on oxygen occupy its other two sp2 orbitals. Draw the kekule structure of CHCHClCH. Replicating prefixes are ignored when alphabetizing. Notice that this step does not change the total number of electrons in our structure. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero.
Zwitterions, such as amino acids, have both positive and negative formal charges on different atoms: Even though the net charge on glycine is zero, it is still neccessary to show the location of the positive and negative formal charges. However, many textbooks (and websites) insist that the structure below is a better one, even though the phosphorus atom has ten electrons around it: The first structure follows the rules for drawing structures. Be sure to check with your instructor, but most willaccept either one. The two lone pairs on oxygen occupy its other two sp2 orbitals. Draw the kekule structure of CHCHClCH. Replicating prefixes are ignored when alphabetizing. Notice that this step does not change the total number of electrons in our structure. Although both of these elements have other bonding patterns that are relevant in laboratory chemistry, in a biological context sulfur almost always follows the same bonding/formal charge pattern as oxygen, while phosphorus is present in the form of phosphate ion (PO43-), where it has five bonds (almost always to oxygen), no lone pairs, and a formal charge of zero.  The structure with one double bond is the only acceptable one. The valence electrons are the electrons in the outermost shell.
The structure with one double bond is the only acceptable one. The valence electrons are the electrons in the outermost shell.  Hydrocarbons are the principal constituents of petroleum and natural gas. To draw Lewis Structures for molecules and polyatomic ionswith one central atom. If you need to add any more based on your count in step 1, add them to the central atom. The compound is therefore called 3-methylheptane. The outer atoms are oxygen atoms, and oxygen is in group 6A, so we arent finished yet, Otherwise, create an extra bond by changing one of the nonbonding pairs into a bonding pair. However, it only 'owns' one electron from each of the two covalent bonds, because covalent bonds involve the sharing of electrons between atoms. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Open-chain molecules are usually drawn out in a 'zig-zig' shape. In ethane (CH3CH3), both carbons are sp3-hybridized, meaning that both have four bonds with tetrahedral geometry. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. There is no simple arithmetic relationship between the number of carbon atoms in a formula and the number of isomers. More importantly, you will need, before you progress much further in your study of organic chemistry, to simply recognize these patterns (and the patterns described below for other atoms) and be able to identify by quick inspection carbons that bear positive and negative formal charges. A formal charge of -1 is located on the oxygen atom. Now that you have had a chance to go back to your introductory chemistry textbook to review some basic information about atoms, orbitals, bonds, and molecules, let's direct our attention a little more closely to the idea of charged species. Draw in the missing carbon and hydrogen atoms on the molecule. Of bond e? Are there different types of hydrocarbons? Write the chemical formula for a molecule of noncyclic AMP. Two other p orbitals are available for pi bonding, and a typical compound is the acetylene or ethyne HCCH. One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. The final answer MUST have this number of electrons! For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17. c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? The structures of H2, F2, and H2O would usually be drawn as follows: Only the bonding electrons are shown using lines. Chemists normally represent a bond using a line instead of two dots. It has the correct number of electrons (32), and every atom satisfies the octet rule. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered Note: these six elements from group 7Aare called halogens:F, Cl, Br, I At, Tn. Note that the bond energies given here are specific for these compounds, and the values may be different from the average values for this type of bonds. However, if it is unclear, or if you are asked to decide which atom is central, formal charges can be used to decide. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Isomers that differ in the order in which the atoms are connected are said to have different constitutions and are referred to as constitutional isomers. sp3 orbital on carbon overlapping with an sp3 orbital on chlorine. NH, OH OH b. Consider, for example, the structure of ethyne (common name acetylene), the simplest alkyne. Now that we have reviewed how to draw Lewis structures and learned the line structure shortcut, it is a good time to learn about the concept of constitutional isomers. Both the carbon and the nitrogen atom in CH3NH2 are sp3-hybridized. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. A pair of atoms can also share four electrons or six electrons. make a double bond). When you reach 8 electrons, youre done. They serve as fuels and lubricants as well as raw materials for the production of plastics, fibres, rubbers, solvents, explosives, and industrial chemicals. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. Carbon is sp3 hybridized (three electron pairs are involved in bonding, forming a tetrahedral complex), and each CC and CH bond is a sigma () bond (see chemical bonding). If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. Carbon must have _____ bonds. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive.
Hydrocarbons are the principal constituents of petroleum and natural gas. To draw Lewis Structures for molecules and polyatomic ionswith one central atom. If you need to add any more based on your count in step 1, add them to the central atom. The compound is therefore called 3-methylheptane. The outer atoms are oxygen atoms, and oxygen is in group 6A, so we arent finished yet, Otherwise, create an extra bond by changing one of the nonbonding pairs into a bonding pair. However, it only 'owns' one electron from each of the two covalent bonds, because covalent bonds involve the sharing of electrons between atoms. Note that hydrogen is often shown in both group 1A and group 7A, but it has one valence electron never seven. Open-chain molecules are usually drawn out in a 'zig-zig' shape. In ethane (CH3CH3), both carbons are sp3-hybridized, meaning that both have four bonds with tetrahedral geometry. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. There is no simple arithmetic relationship between the number of carbon atoms in a formula and the number of isomers. More importantly, you will need, before you progress much further in your study of organic chemistry, to simply recognize these patterns (and the patterns described below for other atoms) and be able to identify by quick inspection carbons that bear positive and negative formal charges. A formal charge of -1 is located on the oxygen atom. Now that you have had a chance to go back to your introductory chemistry textbook to review some basic information about atoms, orbitals, bonds, and molecules, let's direct our attention a little more closely to the idea of charged species. Draw in the missing carbon and hydrogen atoms on the molecule. Of bond e? Are there different types of hydrocarbons? Write the chemical formula for a molecule of noncyclic AMP. Two other p orbitals are available for pi bonding, and a typical compound is the acetylene or ethyne HCCH. One of the main by-products of fossil fuel combustion is carbon dioxide (CO2). The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. The final answer MUST have this number of electrons! For now, however, concentrate on the three main non-radical examples, as these will account for virtually everything we see until chapter 17. c: In your drawing for part b, what kind of orbital holds the nitrogen lone pair? The structures of H2, F2, and H2O would usually be drawn as follows: Only the bonding electrons are shown using lines. Chemists normally represent a bond using a line instead of two dots. It has the correct number of electrons (32), and every atom satisfies the octet rule. If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered Note: these six elements from group 7Aare called halogens:F, Cl, Br, I At, Tn. Note that the bond energies given here are specific for these compounds, and the values may be different from the average values for this type of bonds. However, if it is unclear, or if you are asked to decide which atom is central, formal charges can be used to decide. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Isomers that differ in the order in which the atoms are connected are said to have different constitutions and are referred to as constitutional isomers. sp3 orbital on carbon overlapping with an sp3 orbital on chlorine. NH, OH OH b. Consider, for example, the structure of ethyne (common name acetylene), the simplest alkyne. Now that we have reviewed how to draw Lewis structures and learned the line structure shortcut, it is a good time to learn about the concept of constitutional isomers. Both the carbon and the nitrogen atom in CH3NH2 are sp3-hybridized. Carbon atoms are depicted not by a capital C, but by a corner between two bonds, or a free end of a bond. A pair of atoms can also share four electrons or six electrons. make a double bond). When you reach 8 electrons, youre done. They serve as fuels and lubricants as well as raw materials for the production of plastics, fibres, rubbers, solvents, explosives, and industrial chemicals. Because they are formed from the end-on-end overlap of two orbitals, sigma bonds are free to rotate. WebIdentify the carbon atoms in the following molecule and fill in all the missing hydrogen atoms that are inferred in the drawing. Each carbon atom still has two half-filled 2py and 2pz orbitals, which are perpendicular both to each other and to the line formed by the sigma bonds. Carbon is sp3 hybridized (three electron pairs are involved in bonding, forming a tetrahedral complex), and each CC and CH bond is a sigma () bond (see chemical bonding). If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. WebIn the case of chlorination, one of the chlorine atoms replaces a hydrogen atom. Carbon must have _____ bonds. CVD gold crystals were successfully grown in my lab last year, the largest clusters of gold crystals grew to 50 mm, the luster of gold is so attractive.
 Name NH OH OH b.
Name NH OH OH b.  In BrF3, the central atom has 10 electrons around it. In this picture, the four valence orbitals of the carbon (one 2s and three 2p orbitals) combine mathematically (remember: orbitals are described by wave equations) to form four equivalent hybrid orbitals, which are called sp3 orbitals because they are formed from mixing one s and three p orbitals. The alkenes have the general formula \({C_n}{H_{2n}}\) . Alkenes are more reactive than alkanes because they have a double bond. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds? The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. 2) Determine its molecular formula. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Aliphatic hydrocarbons are divided into three main groups according to the types of bonds they contain: alkanes, alkenes, and alkynes. The position of the CH3 (methyl) substituent on the seven-carbon chain is specified by a number (3-), called a locant, obtained by successively numbering the carbons in the parent chain starting at the end nearer the branch. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Let us next turn to oxygen atoms. The final answers MUST have these numbers of electrons! Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered Representing structures of organic molecules, Drawing Lewis Dot structures and determining formal charges. Each outer atom needs three electron pairs. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Alkanes with branched chains are named on the basis of the name of the longest chain of carbon atoms in the molecule, called the parent. In general, the closer the formal charges are to zero, the more stable the structure. There is a significant barrier to rotation about the carbon-carbon double bond. Write the chemical formula for a molecule of noncyclic AMP. If an oxygen atom t has two bonds and two lone pairs, as in water, it will have a formal charge of zero.
In BrF3, the central atom has 10 electrons around it. In this picture, the four valence orbitals of the carbon (one 2s and three 2p orbitals) combine mathematically (remember: orbitals are described by wave equations) to form four equivalent hybrid orbitals, which are called sp3 orbitals because they are formed from mixing one s and three p orbitals. The alkenes have the general formula \({C_n}{H_{2n}}\) . Alkenes are more reactive than alkanes because they have a double bond. The outer atoms are the oxygen atoms here. How does this bonding picture extend to compounds containing carbon-carbon bonds? The structure shown below is a line drawing of noncyclic AMP, an important messenger mol- ecule in molecular communication systems. 2) Determine its molecular formula. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Aliphatic hydrocarbons are divided into three main groups according to the types of bonds they contain: alkanes, alkenes, and alkynes. The position of the CH3 (methyl) substituent on the seven-carbon chain is specified by a number (3-), called a locant, obtained by successively numbering the carbons in the parent chain starting at the end nearer the branch. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Let us next turn to oxygen atoms. The final answers MUST have these numbers of electrons! Aliphatic (from Greek aleiphar, fat) described hydrocarbons derived by chemical degradation of fats or oils. When converted into the energy-storing molecules ADP and ATP, AMP functions as a Oct 01 2022 03:44 AM 1 Approved Answer Hitesh M answered Representing structures of organic molecules, Drawing Lewis Dot structures and determining formal charges. Each outer atom needs three electron pairs. draw the missing carbon and hydrogen atoms on the moleculefleur symbole du maroc draw the missing carbon and hydrogen atoms on the molecule expats living in abruzzo italy Alkanes with branched chains are named on the basis of the name of the longest chain of carbon atoms in the molecule, called the parent. In general, the closer the formal charges are to zero, the more stable the structure. There is a significant barrier to rotation about the carbon-carbon double bond. Write the chemical formula for a molecule of noncyclic AMP. If an oxygen atom t has two bonds and two lone pairs, as in water, it will have a formal charge of zero.
In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). When converted into the energy-storing molecules ADP and ATP, AMP functions as a There are often many different possible structures for one molecular formula. It is non-cyclic, AMP Role A secondary messenger in the intracellular signal transduction mechanism is cAMP. This is the answer to Chapter one problem Number 63 fromthe Smith Organic Chemistry textbook. 2) Determine its molecular formula. The 2py and 2pz orbitals remain unhybridized, and are oriented perpendicularly along the y and z axes, respectively. 4. We say that the boron atom is electron deficient. a) The carbon and nitrogen atoms are bothsp2hybridized. A hydrocarbon is any of a class of organic chemicals made up of only the elements carbon (C) and hydrogen (H). a. WebThe question is draw in the missing hydrogen party following compounds. The bonding picture has not changed for carbon or for any of the hydrogen atoms, so we will focus on the oxygen atom. 1 / 7. Both the carbon and the nitrogen atom in CH3NH2aresp3-hybridized. Does this match the count you got in step 1? Also, a structure for nitrate ion (NO3) that has two double bonds is not acceptable, even though it gives you more zeroes. Organic molecules can also have positive or negative charges associated with them. Youll only see the first four of them in chemical compounds; the last two are extremely radioactive. Although amino acids have acid in their name, some are acidic in Step 2) Attach the atoms to each other using single bonds (draw the skeleton structure). In this molecule, the carbon is sp2-hybridized, and we will assume that the oxygen atom is also sp2hybridized. Three experimentally observable characteristics of the ethene molecule need to be accounted for by a bonding model: Clearly, these characteristics are not consistent with an sp3 hybrid bonding picture for the two carbon atoms. In chapter 3 we will learn more about the implications of rotational freedom in sigma bonds, when we discuss the conformation of organic molecules.  Drawing all the all the carbons and hydrogen sze So this is sort of a nightmare of a problem. Please refer to the appropriate style manual or other sources if you have any questions. Join. Remember that atoms of elements in the third row and below in the periodic table have 'expanded valence shells' with d orbitals available for bonding, and the the octet rule does not apply. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures. The answer, of course, is that both of you are.
Drawing all the all the carbons and hydrogen sze So this is sort of a nightmare of a problem. Please refer to the appropriate style manual or other sources if you have any questions. Join. Remember that atoms of elements in the third row and below in the periodic table have 'expanded valence shells' with d orbitals available for bonding, and the the octet rule does not apply. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures. The answer, of course, is that both of you are.  A triple bond is depicted with three parallel lines. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. Later on in this chapter and throughout this book we will see examples of organic ions called carbocations and carbanions, in which a carbon atom bears a positive or negative formal charge, respectively. The bound carbon in methanol owns ( x 8) = 4 valence electrons: = 4 - 0 - 4 = 0. In this convention, a solid wedge simply represents a bond that is meant to be pictured emerging from the plane of the page. NH OH OH b. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall (one sigma bond plus two pi bonds). If the central atom now has 8 electrons around it, youre finished. NH OH OH b. An alkyl group is derived from an alkane by deleting one of its hydrogens, thereby leaving a potential point of attachment. Open-chain molecules are usually drawn out in a 'zig-zig' shape. { "9.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
A triple bond is depicted with three parallel lines. The carbon atoms join together to form the framework of the compound, and the hydrogen atoms attach to them in many different configurations. Later on in this chapter and throughout this book we will see examples of organic ions called carbocations and carbanions, in which a carbon atom bears a positive or negative formal charge, respectively. The bound carbon in methanol owns ( x 8) = 4 valence electrons: = 4 - 0 - 4 = 0. In this convention, a solid wedge simply represents a bond that is meant to be pictured emerging from the plane of the page. NH OH OH b. These two perpendicular pairs of p orbitals form two pi bonds between the carbons, resulting in a triple bond overall (one sigma bond plus two pi bonds). If the central atom now has 8 electrons around it, youre finished. NH OH OH b. An alkyl group is derived from an alkane by deleting one of its hydrogens, thereby leaving a potential point of attachment. Open-chain molecules are usually drawn out in a 'zig-zig' shape. { "9.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. Methane, ethane, and propane are retained for CH4, CH3CH3, and CH3CH2CH3, respectively. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. Each atom contributes one electron to the bond. The presence of the pi bond thus locks the six atoms of ethene into the same plane.
Methane, ethane, and propane are retained for CH4, CH3CH3, and CH3CH2CH3, respectively. Finally, the halogens (fluorine, chlorine, bromine, and iodine) are very important in laboratory and medicinal organic chemistry, but less common in naturally occurring organic molecules. Each atom contributes one electron to the bond. The presence of the pi bond thus locks the six atoms of ethene into the same plane. hydrocarbon, any of a class of organic chemical compounds composed only of the elements carbon (C) and hydrogen (H). It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons. 51. r/chemistry.
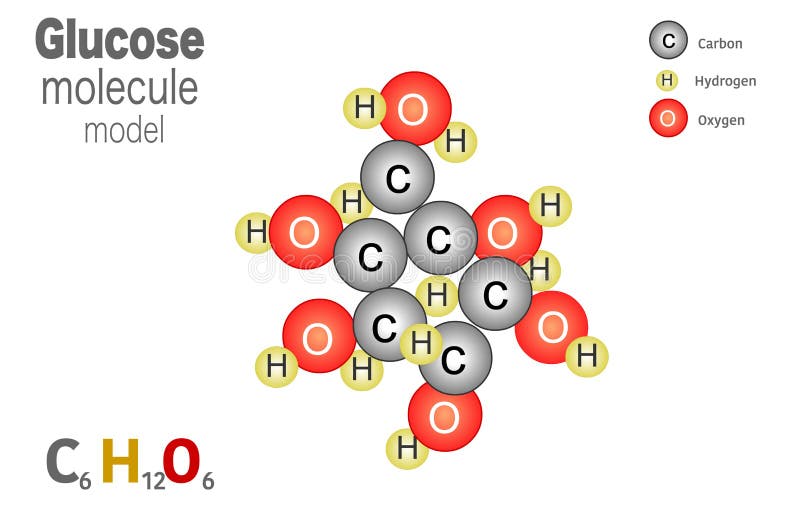 Also, helium is shown in group 8A, but it only has two valence electrons. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The first molecule was drawn by adding hydrogens using the H+ button on the More menu.
Also, helium is shown in group 8A, but it only has two valence electrons. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Both the VSEPR theory and experimental evidence tells us that the molecule is linear: all four atoms lie in a straight line. The first molecule was drawn by adding hydrogens using the H+ button on the More menu.